Thermodynamics
We study the thermodynamic properties of supercritical fluids and their mixtures. Knowledge of the high-pressure phase behaviour is essential in any application using scCO2.
In our labs we have a high-pressure variable volume view cell to measure phase diagrams. With this cell we have measured both high-pressure vapour-liquid equilibirum and solid-fluid equilibrium (see Figure 1).

Figure 1. Interior of the cell with different samples.
Briefly, the cell consists of a high-pressure cell made of stainless steel and fitted with a sapphire window whose volume can be varied by a piston (maximum working volume 15 mL). The contents in the cell can be illuminated and observed through this window using a Fiegert Endotech boroscope and a digital camera connected to a computer. The cell can be heated up to 100 ºC and compressed up to 30 MPa. A scheme of this installation is shown in Figure 2.
Solubility data are measured following the synthetic method. A mixture of known composition is prepared in the cell and its phase behaviour is studied against pressure and temperature. Every component is separately loaded into the cell and their amounts are determined by weight. Liquid carbon dioxide is gravimetrically transferred into the cell by means of an auxiliary cell. Once the sample is loaded, the cell is kept at a constant temperature and the sample is compressed to a single phase. The contents of the cell are agitated by a magnetic stirrer in order to assure homogeneity in the single phase. The pressure is then slowly decreased until the second phase appears. The sample can be alternatively solubilized and precipitated to obtain a precise pressure value.
With this device, we have measured the solubility of different metallorganic compounds and metals salts in supercritical fluids as well as fatty acids and other bioactive pure compounds.
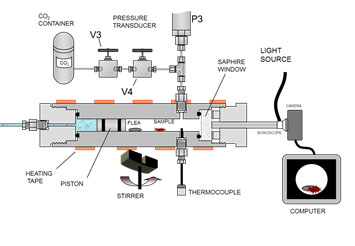
Figure 2. Scheme of the variable view cell
