Supercritical Fluid Extraction (SFE)
Of all the supercritical fluids that have been studied, carbon dioxide (CO2) is the most used due to its low critical temperature (TC = 31ºC) and pressure (PC = 74 bar), non-toxicity, availability and low cost. It is a "green" solvent found in the atmosphere, in food and beverages and of which no minimum content needs to be set in the extracts, so it can be used safely. In fact, it is considered a GRAS solvent.
All supercritical fluid extraction (SFE) processes consist of two stages (see scheme on Figure 1). First, the supercritical CO2 flows through the raw material and dissolves the extractable components. Then, the solvent loaded with the extract is evacuated from the extractor and fed to the separator, where the pressure is reduced so that the solute is not soluble and precipitates. Alternatively, the solute can be separated from the supercritical solvent by adsorption, absorption or with a membrane. In this case, the solvent circuit can be operated at almost constant pressure.
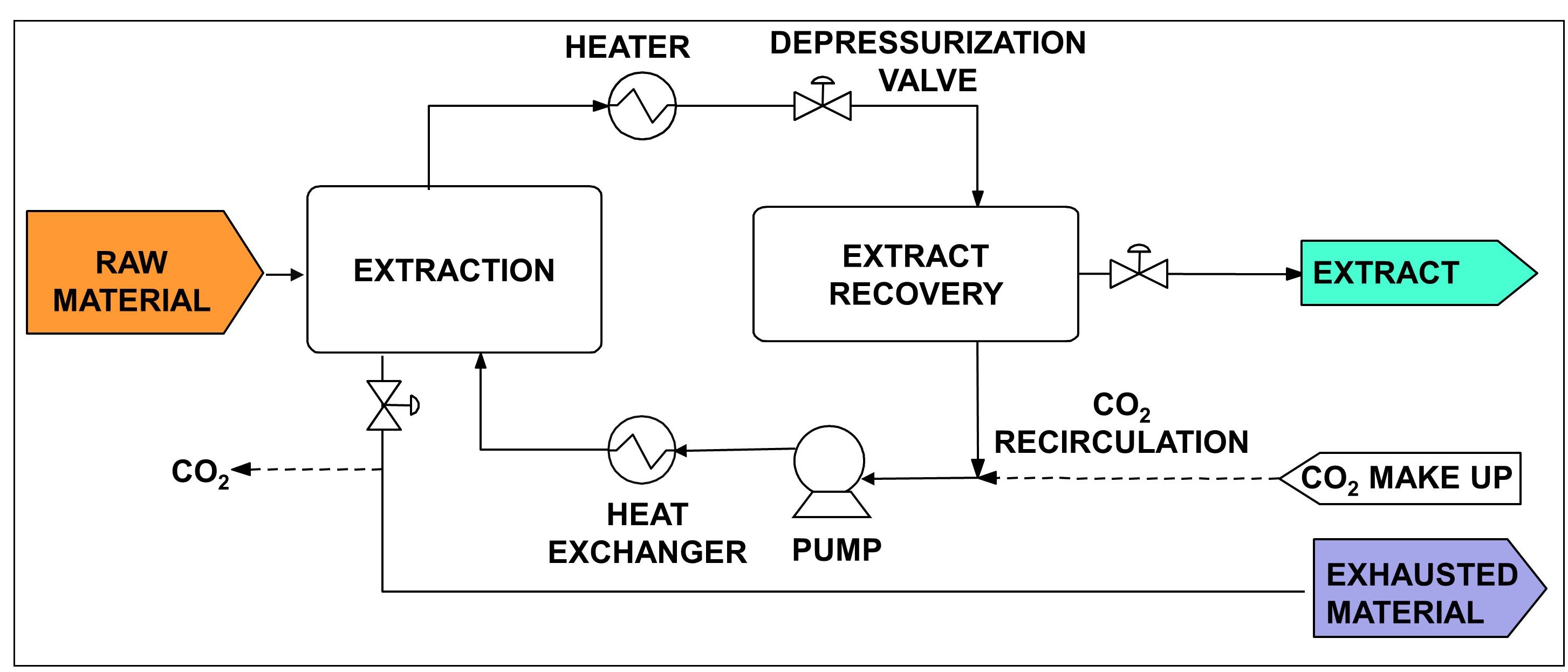
Figure 1. Block diagram of a SFE process.
Generally, a pre-treatment of the solid material is required. The addition of water to facilitate the extraction is frequent. This amount varies between 10 and 40%. If the extracted solute is the product of interest, the raw material may require grinding to increase the yield and speed of extraction.
In general terms, supercritical CO2 extraction processes are run at temperatures of 35-40°C, except in processes where the solute is strongly adsorbed. In those cases, higher temperatures are required to assist in desorption (up to 80°C). The pressure varies between 100 and 500 bar, depending on the type of extract.
Advantages
1. After extraction, CO2 can be removed in gaseous form without leaving no residues in the matrix or in the extract;
2. The aroma and taste of the extracts are more natural;
3. The process is faster, more selective and easier than with organic solvents. Hence, the subsequent purification process is simpler or even unnecessary, compared to conventional extractions which require complex downstream.
4. The solvent is easily recovered and recirculated. Therefore, supercritical extraction can be considered an intensive process.
Given the high costs of the installations, the supercritical extraction is a process suitable for high production and/or relatively high unit selling price. There is a high technological maturity and any application could be taken to an industrial scale in a short period of time.
Experience and Capabilities
We do have experience on supercritical extraction of oils rich in bioactive compounds from plants, microalgae and residues for their revalorization; extraction of caffeine from coffee grounds; oleorresin from paprika; cleaning of metal parts; production of aroma-free cork; cocoa defatting…We are now working on hemp.
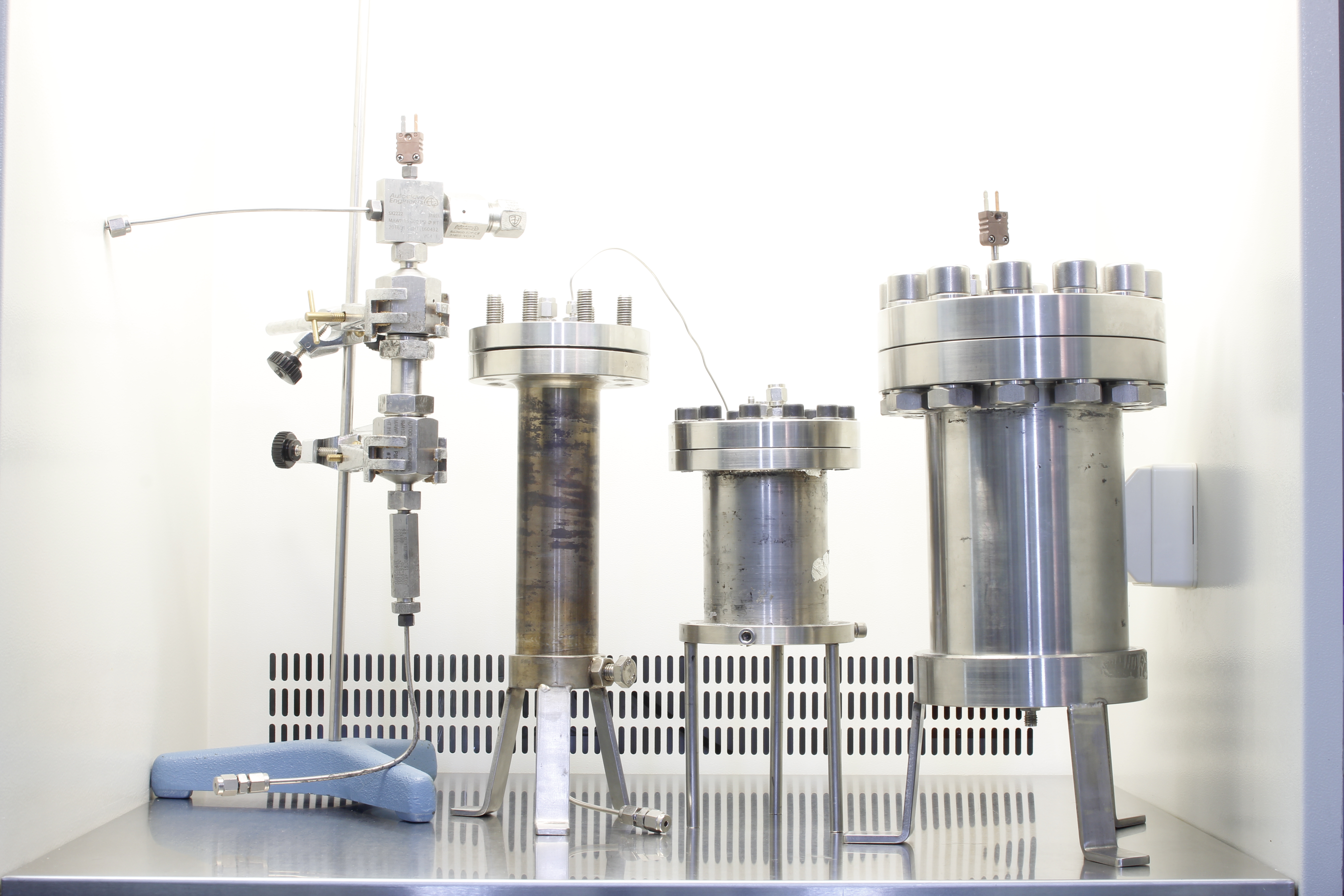
We have facilities of different sizes to carry out extraction tests on all types of materials (Figure 2) and for their pre-treatment (Figure 3). We also have the infrastructure to evaluate the physical-chemical and microbiological quality of the extracts and the exhausted raw materials. We can model the process to make larger scale designs.
Figure 2. Image of some of our extractors. Volume up to 500 mL.
Figure 3. Different raw materials for extraction after milling and sieving.