Supercritical Fluids
At temperatures above 31 ºC and pressures greater than 7.4 MPa, CO2 becomes a supercritical fluid. Supercritical CO2 has densities intermediate between those of liquids and gases, but transport properties (diffusivity and viscosity) similar to gases.
Figure 1 shows the PT diagram of CO2 , showing the regions of coexistance of solid, liquid, vapour, and supercritical fluid (SCF) phase.
CO2 phase diagram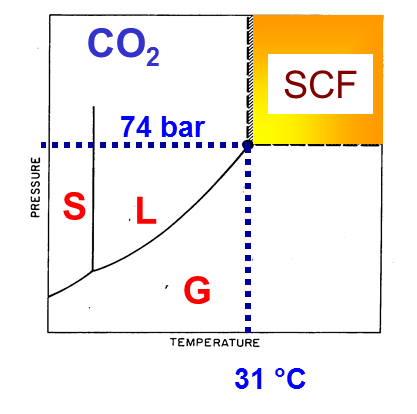
All these properties and the low surface tension of CO2 with solid surfaces allow to introduce metal precursors dissolved in CO2 inside porous materials in a very efficient way. Furthermore, the properties of CO2 can be tuned through small changes of pressure and temperature, changing the final properties of the material.
CO2 is considered a green solvent because it has moderate critical parameters, it is cheap, non-toxic, inert, nonflammable and it can be recycled. Furthermore, CO2 is a gas at atmospheric pressure that can be easily released from the material by depressurization and it does not leave any residue.
