Sterilization
CO2 applied at pressures between 6 and 15 MPa is effective in the inactivation of bacteria, fungi and viruses, as well as enzymes that cause the deterioration of sensitive materials. The treatment does not require high temperatures (for vegetative forms and enzymes typically less than 45ºC). In sporulated forms, it is necessary to raise the temperature to 80-90ºC or to apply the CO2 with small amounts of additives. In the case of solid products, it is necessary to use wet CO2. Processing time is short (<30 min), and control and operation are simple. CO2 is available and has a low cost. It is a "green" solvent that is found in the atmosphere, in food and beverages and of which no minimum content needs to be set in the treated products (GRAS solvent), so it can be used safely.
The product to be treated is placed inside a high-pressure vessel (see scheme of the procedure on Figure 1). The pressurized and preheated CO2 with the additives is put in contact with the product for a certain time in order to achieve the desired reduction of the biological contamination. Then the vessel is depressurized. The CO2 is removed in the form of a gas. The sterile product without CO2 residues is collected for further packaging.
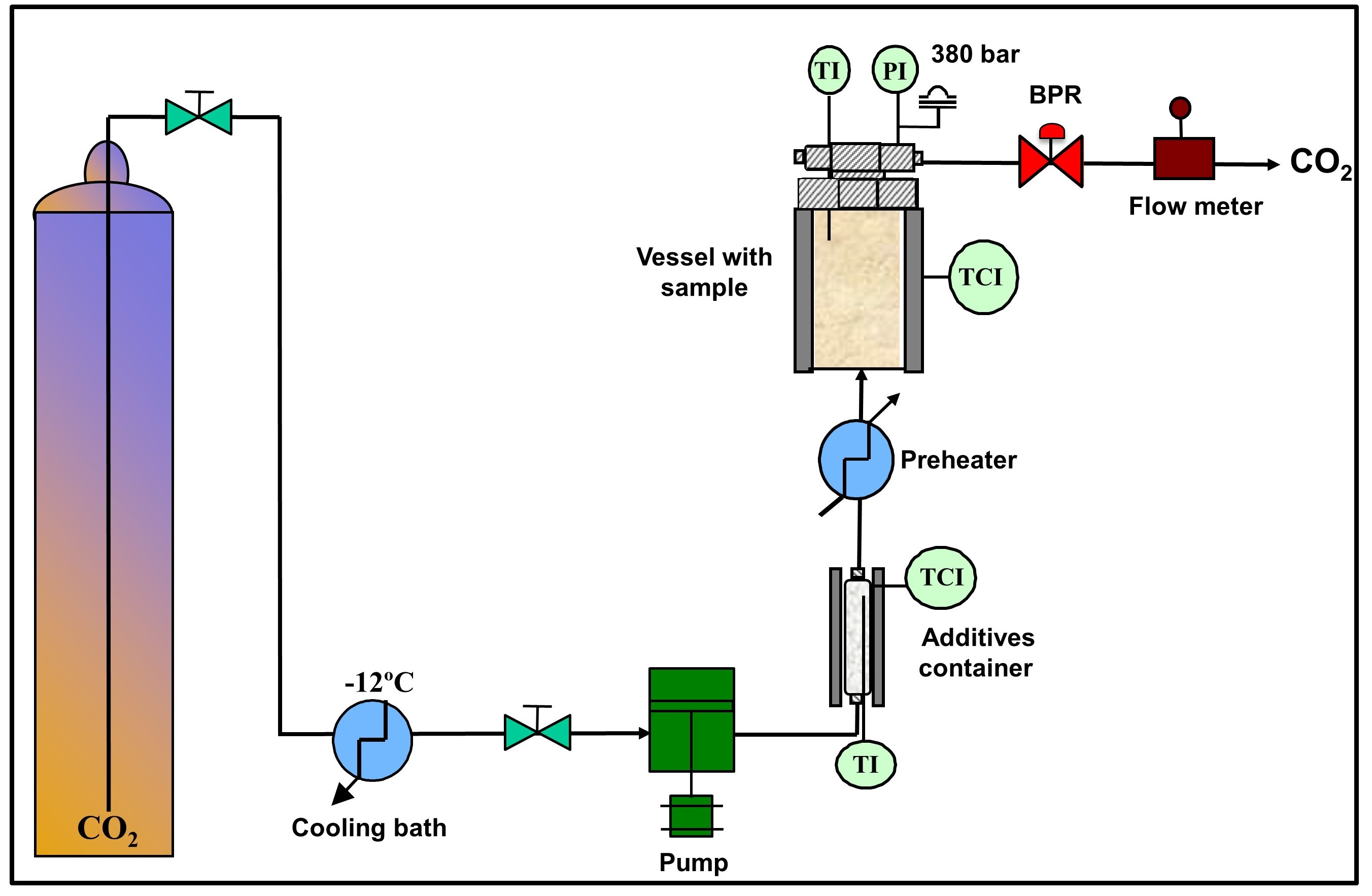
Figure 1. Scheme of a supercritical CO2 sterilization procedure.
Figure 2. One of our members doing microbial tests on the laminar flow chamber.
The method is applicable to sensitive products where other preservation techniques are not effective or appropriate: biomaterials, food, medical materials, biopolymers, pharmaceuticals and cosmetics, textile materials (e.g. hospital or personal protective equipment), electronic and optical devices, etc.
In general, the physicochemical, mechanical, sensory and organoleptic properties of the treated products are little affected by the treatment.
Advantages
- The treated products could be labelled as "natural products"
- Continuous treatment for liquids and continuous-simulated treatment for solids is possible
- It allows to couple sterilization process with extraction and/or drying in a single stage
Experience and Capabilities
We have experience in food products on the elimination of enterobacteria, bacterial and fungal spores, and other typical biocontamination. Also, in the destruction of biological agents in technical clothing and optoelectronic material. Now we are working in biomaterials.
We have vessels of different sizes to carry out concept tests of the supercritical CO2 sterilization procedure on all types of materials, both solid and liquid. We also have the facilities and methodologies to carry out microbiological tests (Figure 2) and to evaluate the physical-chemical quality of the treated products.

