STERILIZATION AND ENZYMATIC DEACTIVATION OF SENSITIVE PRODUCTS THROUGH SUPERCRYTIC CO2
|
Description |

Image of a supercritical CO2 sterilization vessel for laboratory scale solid products.
The most frequently used preservation method is the thermal treatment with steam at 121ºC for 21 min. But this treatment causes alterations in the functional, nutritional and organoleptic properties of the treated products. Chemical preservatives are heavily restricted by legislation and by alterations in the organoleptic properties they cause. Hydrostatic high pressure pasteurization is expensive, does not destroy spores and poses serious safety and installation costs. Gamma irradiation is also expensive and is not well accepted by consumers. Other methods used in medical materials such as ethylene oxide or hydrogen peroxide are very aggressive chemically.
High pressure CO2 is an alternative conservation operation. On the one hand this fluid has low values of viscosity and diffusion coefficient. Its surface tension is equal to zero so it penetrates very well in solid materials. On the other hand, applied at pressures between 60 and 150 bar is effective to deactivate bacteria, fungi and viruses both in vegetative and sporulated form, as well as enzymes causing the deterioration of sensitive products. Treatment does not require high temperatures (for vegetative forms and enzymes typically less than 45 ° C). In sporulated forms it is necessary to raise the temperature to 70-90 ° C or to apply the CO2 with small amounts of natural additives. In the case of solid products, it is necessary to use moist CO2. Processing time is short (<30 min) and control and operation are simple. CO2 is available and has low cost. It is a "green" solvent that is found in the atmosphere, in food and beverages and from which no minimum content needs to be set on the treated products (GRAS solvent), so it can be safely used.
Depending on the product, it has been observed that in general the physical-chemical, sensory and organoleptic properties of the treated products are little affected by the treatment.
It is applicable to sensitive products where other preservation techniques are not effective or adequate: food, medical materials, biopolymers, pharmaceuticals and cosmetics, textiles (eg hospital or Personal Protective Equipment), electronic and optical devices, etc.
|
How does it work |
The product to be treated is placed inside a high pressure vessel. Pressurized and preheated CO2 is brought into contact with the product for a certain time to achieve the reduction of the desired biological contamination. The vessel is then depressurized. The CO2 is removed as a gas. The sterile product without CO2 residues is collected for later packaging.
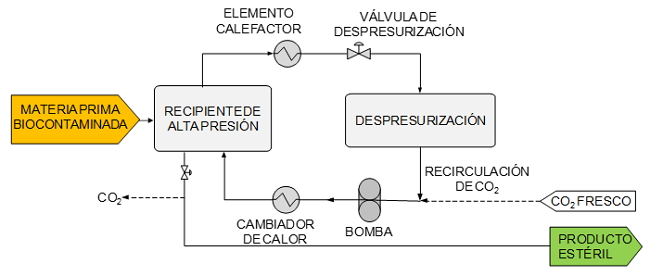
Schematic of a process of sterilization with supercritical CO2 with recirculation of CO2.
|
Advantages |
- Treated products could be labeled as "natural products"
- It is applicable to acid products, such as fruit or canned products
- Continuous liquid treatment and continuous-simulated solid treatment possible
- It allows to couple sterilization process with extraction and / or drying in a single stage
|
Where has it been developed |
The technology has been developed in the group of Supercritical Fluid Processes of the Department of Chemical Engineering. It has been validated in different food products and biomaterials. Also in the destruction of biological agents in technical clothing and optoelectronic material.
|
And also |
|
Contact |
|
© Office for the Transfer of Research Results – UCM |
|
PDF Downloads |
|
Classification |
|
Responsible Researchers |
Lourdes Calvo Garrido: lcalvo@ucm.es
Department: Chemical Engineering
Faculty: Chemical Sciences


