ALLERGENS: ISOLATION AND CHARACTERIZATION. PRODUCTION OF RECOMBINANT ALLERGENS. DIAGNOSTIC TOOLS IN PRECISION MEDICINE
|
Description |
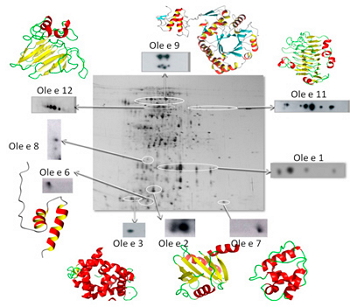
Figure 1. Identification of a bidimensional electrophoresis of the allergens of olive pollen.
Identification, isolation and characterization allergens. Allergy is a major health problem. Its incidence is constantly growing and nowadays affects about 30% of the population in developed countries. Scientific contributions made by our group, about the identification and isolation of pure and homogeneous allergens to replace conventional allergenic extracts, have helped to improve significantly the diagnosis by allowing identifying the molecules responsible for the allergic reaction and then design a better treatment to treat it. We have more than 30 pure allergens that have allowed assessing the causative agent of sensitization and clinical symptoms of patients, and the likelihood that through these molecules can progressively lead to more sensitizations (a phenomenon known as cross-reactivity). This phenomenon results in a very complex vision (allergy to numerous allergen sources) of a very simple problem (sensitization to a single protein). In the field of food allergy, techniques for detecting hidden allergens in processed foods, such as those from mustard or peanut, or for identifying specific allergens in different tissues of fruits and vegetables, for example in tomato or kiwi seeds, are future approaches. The presence of these molecules cause unexpected reactions between allergic population and sometimes the standardized allergen extracts commercially available lack in the right proportion of these allergens driving to the wrong diagnosis of these patients. Pollens, at different seasons are also a very common source of respiratory allergies. Once entering the airways, they can cause unpleasant and sometimes severe symptoms. Our research group in a translational research has contributed with more than 20 pollen allergens from olive, ash, and Lamb's quarters and Salsola to be used as diagnostic tools.
Production of recombinant allergens. Another important objective in our research is to obtain homogeneous allergenic molecules of high quality and in high amount, to introduce and use them clinically in the pharmaceutical market. Molecular biology and genetic engineering techniques enabled us to produce recombinant allergens in harmless and simple systems such as bacteria or yeast. Thus, we can scale up the production and get several hundred of milligrams of a particular allergen. However, it is essential to carry out the validation of the quality of these recombinant molecules confirming their equivalence to the natural protein derived from pollen or fruit.
Use of phage libraries (Phage Display) and protein microarrays. This powerful screening technology has added substantial advantages to the research in the field of allergy and other pathologies such as cancer and allowed mapping IgE and IgG epitopes and even identifying allergenic proteins, difficult to detect by other techniques. These approaches combined with other omics technologies enabled us to complete the panels of allergens of sometimes extremely complex, clinically relevant sources.
|
How does it work |
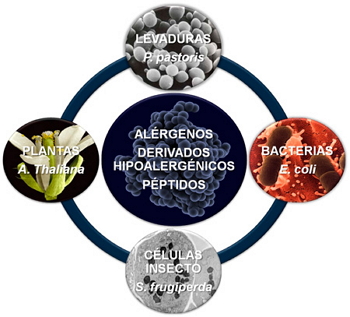
Figure 2. Expression systems used to produce recombinant proteins.
The proteins responsible of the patient’s sensitization are purified from their biological sources -pollens and plant foods-. At our laboratory, we have great experience of over 30 years in both protein purification and characterization using conventional chromatographic systems or more sophisticated ones as HPLC or FPLC. By means of two-dimensional electrophoresis (Figure 1), we can determine the molecular mass, the isoelectric point and the polymorphism degree from a given allergen.
The messenger RNA encoding a particular protein is isolated and the corresponding complementary DNA (cDNA) obtained. Then, the cDNA encoding the allergen of interest was amplified by PCR is cloned into a vector that -once amplified into a host cell- works as expression vehicle for recombinant protein production. The transformed cells with the vector, (usually bacteria –Escherichia coli-, yeast –Pichia pastoris-, insect cell-via Baculovirus infection– or plants -Arabidopsis thaliana-) (Figure 2), are grown till reaching a suitable production levels for the recombinant protein.
To facilitate different stages of the process, induction and subsequent isolation, is convenient to produce the DNA of the target protein fused with one encoding other polypeptide chain – the whole protein or a peptide fragment-. In this case, a fusion protein is produced. Later on, the protein is purified to homogeneity, using its physicochemical properties including molecular mass, solubility, charge, etc.
To assess that the recombinant protein effectively replaces the purified allergen isolated from the natural biological source, is essential to verify that their molecular features are equivalent. To that end, the structural and functional characterization of the recombinant protein is a critical step when evaluating the expression system.
|
Advantages |
The recombinant DNA expression system provides an unlimited production of highly homogeneous proteins whose presence in nature is poorly represented or, in any case, its isolation involves important difficulties. They can also be prepared, by site-directed mutagenesis, derivatives of these molecules with higher stability or modified biological activity properties.
|
Where has it been developed? |
These technologies have been developed in the Department of Biochemistry and Molecular Biology, Chemistry Faculty of Universidad Complutense de Madrid. We are leading our group for more than 30 years producing proteins either purified from biological sources or recombinant ones. More than 20 proteins have been produced in E. coli, and over 15 in P. pastorisand Baculovirus expression systems. These allergens have been purified, and the analysis of their structural and functional properties has shown to be equivalent to the corresponding forms produced in nature. The group has more than 160 publications in journals of medium-high impact index in the field of Biochemistry and molecular Biology, Biotechnology, Immunology and Biomedicine. Among them, New England Journal of Medicine, Journal of Allergy and Clinical Immunology, Journal of Immunology, Journal of Biological Chemistry, PNAS, Allergy, Molecular Immunology, Clinical and Experimental Allergy, Biochemical Journal, FEBS Journal, Protein Science, Journal of Biotechnology, Protein Expression and Purification, FEBS Lett., BBA, Molecular Nutrition and Food Research. There have been presented more than 20 Doctoral Theses and obtained about 20 regional, national and European projects, as well as numerous projects with Companies and Foundations. The group has 7 patents with Spanish license and have received several awards.
|
And also: |
During the last years, allergy has been defined as a dysfunction of the epithelial barrier that works as internal and external environment interface. In the particular case of asthmatic patients, they show an altered respiratory epithelial barrier. The study of how the respiratory epithelial physiological state influences allergic response and which factors act in a synergistic way with those molecules, and the involvement of the pulmonary surfactant –the lipid-protein mixture that cover the pulmonary alveolus- represent another research line that has required setting new techniques of cell cultures and microscopy. As in vitro models of respiratory epithelium, they have been used cultures - in transwell and at air-liquid interface- of normal human bronchial epithelial (NHBE) cells that once they are differentiated, form a pseudostratified epithelium, and Calu-3 and A549 immortalized cell lines. Different techniques are used together with proteomic and transcriptomics, to obtain a differentially profile of proteins, mRNA and miRNA, allowing us to get a deeper knowledge of allergy response against aeroallergens, in order to design new therapeutic strategies for allergy treatment.
Our possible collaborative role would focus to adapt these systems to customer needs, that is, designing protocols for the isolation of the proteins according to the quality or quantity requirements we are required. Production, mutagenesis and obtaining modified products, analogous to the natural protein that meet specific customer needs. Technical assessment for the incorporation of the methodology to the partner firm.
The design of the corresponding primers from the complete or partial sequence of a protein obtained from biological material to synthesize cDNA. Choice of the expression system for this cDNA, according to the particular structural properties of the target protein, presence of disulfide bonds, folding, etc. Development process of expression. Isolation of expressed recombinant protein, making use of its physicochemical properties: molecular weight, solubility, charge, hydrophobicity, functional affinity. Structural and functional characterization of the recombinant protein and assessment of their equivalence to the natural form of the protein isolated from the biological source, as proof of its viability.
|
Contact |
|
© Office for the Transfer of Research Results – UCM |
|
PDF Downloads |
|
Classification |
|
Responsible Researcher |
Mayte Villalba Díaz: mvillalb@ucm.es
Department: Biochemistry and Molecular Biology I
Faculty: Chemical Sciences


