2015
A Versatile Synthesis of β‐Lactam‐Fused Oxacycles through the Palladium‐Catalyzed Chemo‐, Regio‐, and Diastereoselective Cyclization of Allenic Diols. B. Alcaide, P. Almendros, R.Carrascosa, L. Casarrubios, E. Soriano. Chem. Eur. J. 2015, 21, 2200-2213.
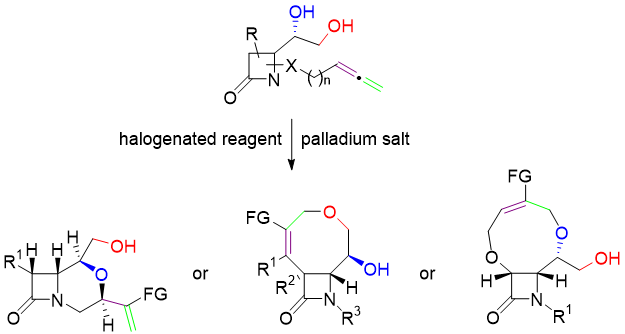
Acid‐Catalyzed Synthesis of α,β‐Disubstituted Conjugated Enones by a Meyer–Schuster‐Type Rearrangement in Allenols. B. Alcaide, P. Almendros, S. Cembellín, T. Martínez del Campo. Adv. Synth. Catal. 2015, 357, 1070-1078.
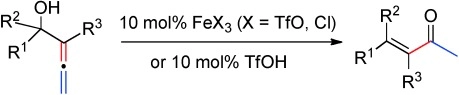
An Alternative to Precious Metals: Hg(ClO4)2·3H2O as a Cheap and Water-Tolerant Catalyst for the Cycloisomerization of Allenols. B. Alcaide, P. Almendros, A. Luna, E. Soriano. J. Org. Chem. 2015, 80, 7050-7057.
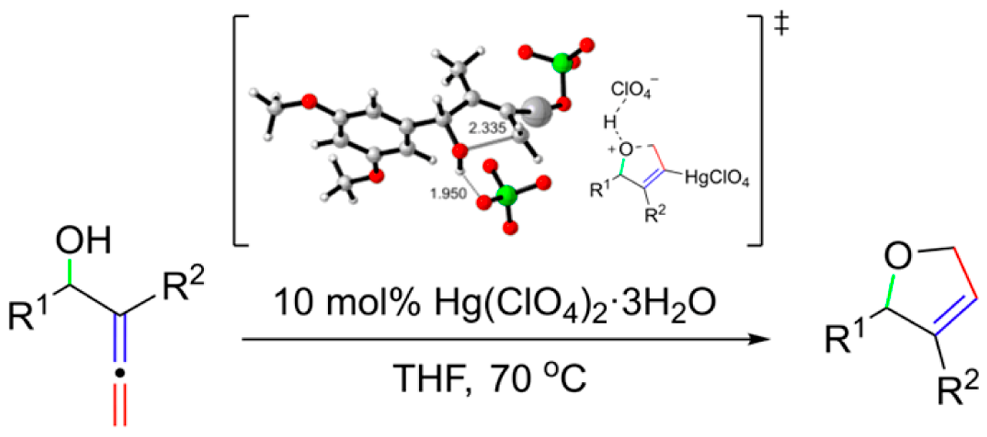
Divergent Reactivity of Homologue ortho‐Allenylbenzaldehydes Controlled by the Tether Length: Chromone versus Chromene Formation. B. Alcaide, P. Almendros, I. Fernández, T. Martínez del Campo, T. Naranjo. Chem. Eur. J. 2015, 21, 1533-1541.
Gallium-Catalyzed Domino Arylation/Oxycyclization of Allenes with Phenols. B. Alcaide, P. Almendros, F. Herrera, A. Luna, M. Elena de Orbe, M. Rosario Torres. J. Org. Chem. 2015, 80, 4157-4163.
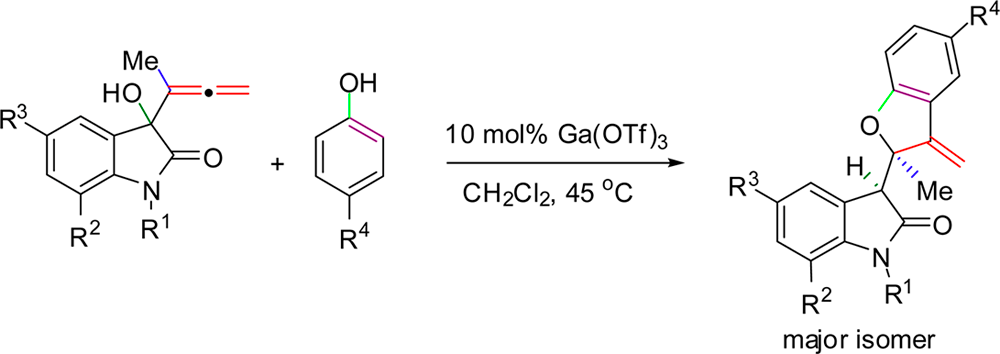
Gold as Catalyst for the Hydroarylation and Domino Hydroarylation/N1–C4 Cleavage of β-Lactam-Tethered Allenyl Indoles. B. Alcaide, P. Almendros, S. Cembellín, T. Martínez del Campo. J. Org. Chem. 2015, 80, 4650-4660.
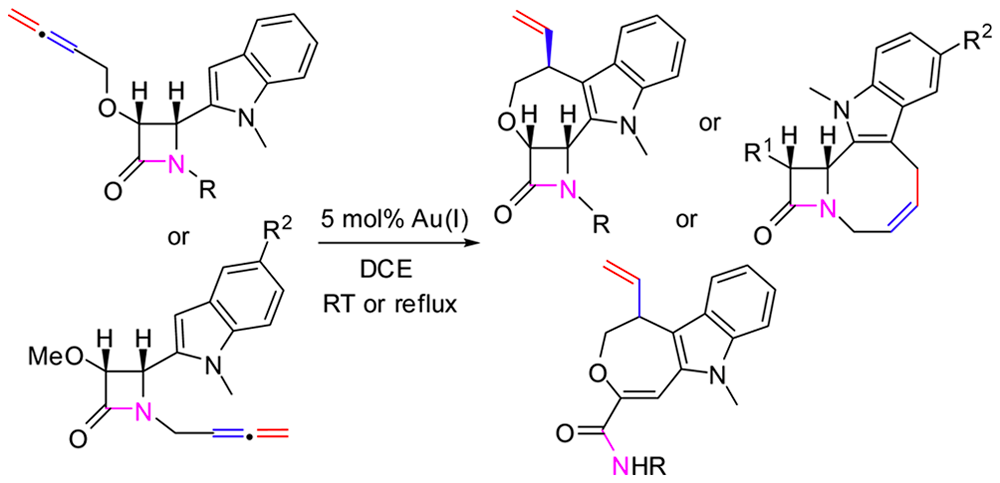
Gold-Catalyzed Reactivity Reversal of Indolizidinone-Tethered β-Amino Allenes Controlled by the Stereochemistry. B. Alcaide, P. Almendros, I. Fernández, R. Martín-Montero, F. Martínez-Peña, M. Pilar Ruiz, M. Rosario Torres. ACS Catal. 2015, 5, 4842-4845.
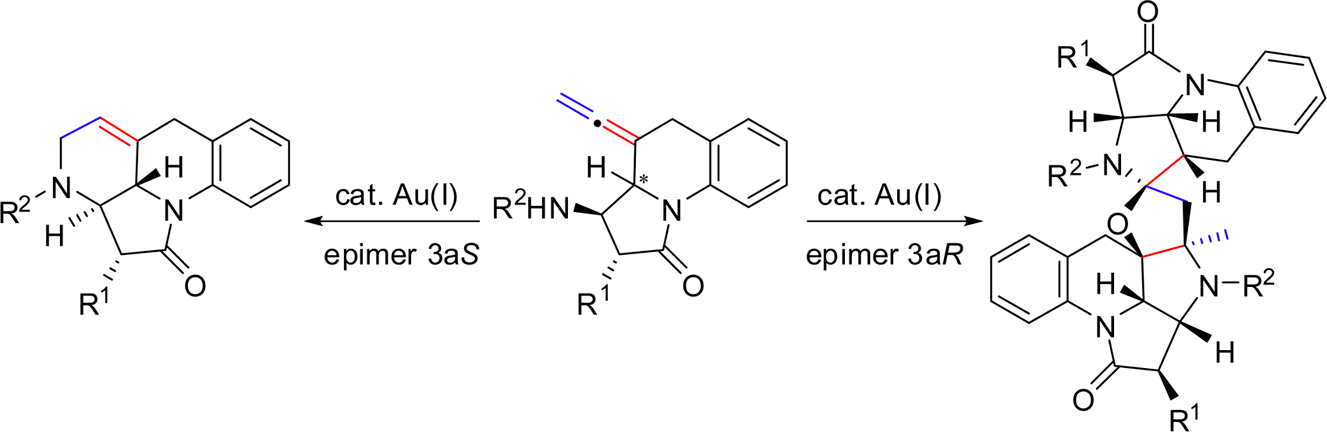
Investigation of the Passerini and Ugi reactions in β-lactam aldehydes. Synthetic applications. B. Alcaide, P. Almendros, C. Aragoncillo, R. Callejo, M. Pilar Ruiz, M. Rosario Torres. Org. Biomol. Chem. 2015, 13, 1387-1394.
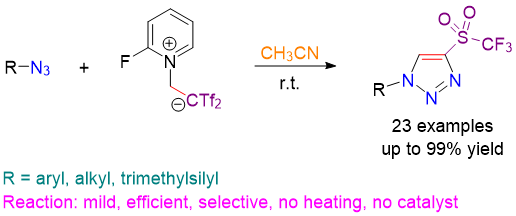
Metal-free [3+2] cycloaddition of azides with Tf2C=CH2 for the regioselective preparation of elusive 4-(trifluoromethylsulfonyl)-1,2,3-triazoles. B. Alcaide, P. Almendros, C. Lázaro-Milla. Chem. Commun. 2015, 51, 6992-6995.
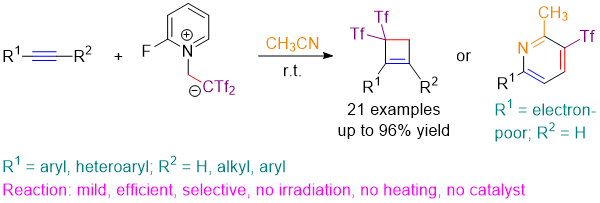
Unveiling the uncatalyzed reaction of alkynes with 1,2-dipoles for the room temperature synthesis of cyclobutenes. Benito Alcaide, Pedro Almendros, Israel Fernández, C. Lázaro-Milla. Chem. Commun. 2015, 51, 3395-3398.
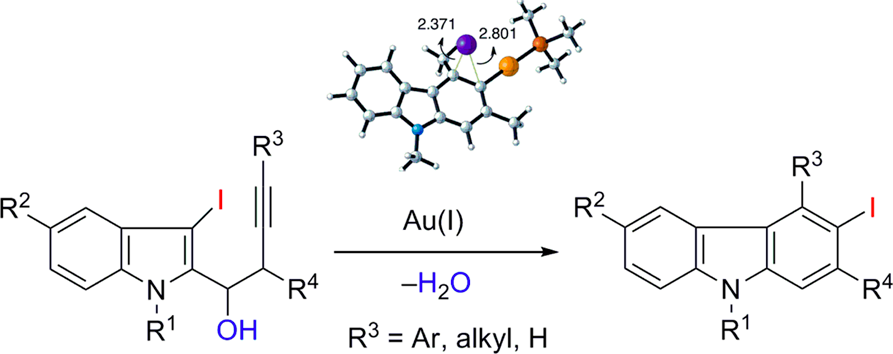
Versatile Synthesis of Polyfunctionalized Carbazoles from (3-Iodoindol-2-yl)butynols via a Gold-Catalyzed Intramolecular Iodine-Transfer Reaction. B. Alcaide, P. Almendros, J. M. Alonso, E. Busto, I. Fernández, M. Pilar Ruiz, G. Xiaokaiti. ACS Catal. 2015, 5, 3417-3421.
Ring Expansions of β-Lactams and β-(thio)lactones. Synthesis of 4- to 7-membered Heterocycles by Ring Expansion. Topics in Heterocyclic Chemistry. B. Alcaide, P. Almendros, C. Aragoncillo. Editors: M. D’hooghe, H. J. Ha. Springer International Publishing, 2015, vol. 41, 233-280. (ISBN=978-3-319-24960-5)
Abstract: The skeleton of β-lactams and β-lactones is present in biologically active compounds. For example, the β-lactam ring is present in classical antibiotics such as penicillins and cephalosporins. On the other hand, there are several natural products containing a β-lactone moiety, with interesting inhibitory activity. In addition, both fragments are very useful synthetic intermediates for the preparation of cyclic and acyclic compounds. The ring strain present in both motifs is involved in this versatile reactivity. This chapter is devoted to the synthesis of five- to seven-membered heterocycles by ring expansion of β-lactams and β-lactones. Different methodologies have been described, and the mechanism for the formation of the products has been discussed. In addition, the applicability of some of the processes has been demonstrated by the synthesis of fragments of natural or biologically relevant compounds. The contributions presented in this chapter have been selected mainly from the developments achieved in the last decade.
DOI: https://doi.org/10.1007/7081_2015_153
Chapter 4 - Four-Membered Ring Systems. Progress in Heterocyclic Chemistry. B. Alcaide, P. Almendros. Editors: G. W. Gribble, J. A. Joule. Elsevier, 2015, vol. 27, 97-115. (ISSN=0959-6380, ISBN=9780081000243)
Abstract: This review covers work published in the calendar year 2014. The synthesis and reactivity of four-membered heteroatom-containing cycles are reviewed. New chemistry of these strained heterocycles, especially oxa- and azaheterocycles, is covered.
DOI: https://doi.org/10.1016/B978-0-08-100024-3.00004-0
