STABILITY AND STABILIZATION OF ACTIVE SUBSTANCES, MEDICINES AND COSMETICS, FOR HUMAN AND VETERINARY USE
|
Description |
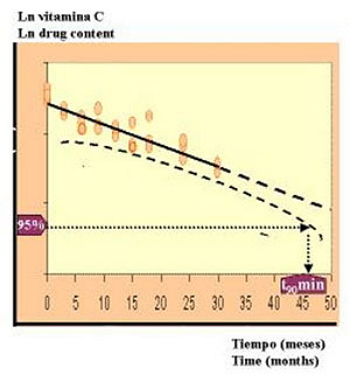
Estimation of the shelf-life of cyanocobalamin injections: minimum t90 according to ICH regulation.
A research group of the University Institute of Industrial Pharmacy of the Complutense University of Madrid offers methodology and equipment to determine the causes of instability of active substances, medicines and cosmetics, propose stabilization resources, estimate shelf lives and do studies of stability according to the regulations required for the registration of such products.
A research group of the University Institute of Industrial Pharmacy of the Complutense University of Madrid offers methodology and equipment to determine the causes of instability of active substances, medicines and cosmetics, propose stabilization resources, estimate shelf lives and do studies of stability according to the regulations required for the registration of such products.
All these aspects are the object of stability studies that can aim to identify the causes of alteration of a product; quantify the degradation of a product under certain circumstances; characterize the degradation kinetics of a product with the possibility of making extrapolations over time; or compare the stability of different formulations. In addition, the offer includes the research and application of different resources for the stabilization of a product and the evaluation of the effectiveness of these resources to improve stability.
|
How does it work |
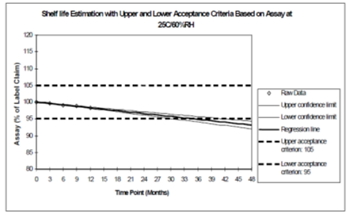
Estimation of the shelf-life with acceptance limits.
During the development of a new drug product or cosmetic it is essential to identify possible causes of instability, which can lead to physical, chemical or microbiological alterations in the final product. At the formulation step, different stabilization resources can be applied in order to avoid or reduce these alterations. The extensive experience in pharmaceutical development of the research group enables them to successfully approach most of the stabilization problems that arise in solid, semi-solid and liquid formulations.
On the other hand, the training and experience of the research group make them possible to carry out a continuous adaptation to the regulation of the European Medicines Agency (EMA), the US Food and Drug Administration (FDA) and the regulation issued by the regulatory authorities of any country in the world, on stability studies and estimation of the shelf-life for the registration of medicines and cosmetics. In this sense, it should be noted that in recent years, international agreements have been reached regarding the requirements and procedures of the stability studies required by the Regulatory Authorities for the commercialization of medicines in the different countries. These agreements, in part, are consequence to the large proliferation, due to absorption and mergers, of large pharmaceutical companies.
Thus the International Committee for Harmonization (ICH) was established, which has developed different guidelines related to the minimum requirements of stability studies of medicinal products for human and veterinary use required for registration and marketing by the authorities of the European Union, the United States and Japan (climatic zones I and II). In practice, these documents have also been adopted by the Regulatory Authorities of third countries, in climatic zones III and IVa mainly. The methodology consists of carrying out different stability studies:
1. - Stress stability studies: Depending on the active principle, the effect of the temperature, and if appropriate, of the humidity, on its stability is determined. The possibility of hydrolytic reactions over a wide range of pH values with the active substance in solution or suspension can also be studied; and the oxidation possibilities of the molecule and the protective effect of different antioxidant agents. Photostability studies are also carried out, obligatory to request the authorization of commercialization of a new drug, evaluating the effect of visible and UV radiation. By these studies the causes of degradation and the degradation products are identified, and the results are considered as supporting data to define the conditions of conservation and the period of validity of a drug or cosmetic.
2. Formal stability studies: The storage conditions and the duration of the studies should cover the distribution, the conservation and the subsequent use of the product. All these conditions are defined in the ICH guideline. Two situations: Preparations to be stored in controlled conditions and preparations to be stored under environmental conditions.
2.1. Long-term stability studies: Its main objective is to determine the shelf life of the product. Therefore, the samples are stored under conditions that represent the expected conditions of storage of the product once marketed. The duration of the stability study should cover the "minimum duration" stipulated by the ICH guidelines. However, when the results of the long-term study included in the documentation do not cover the proposed shelf life, the stability studies should continue after approval covering the "total duration of the study".
2.2. Accelerated and intermediate stability studies. In these studies the samples are stored under conditions that simulate those that could be given during the distribution of the product. Its main objective is to guide the precautions to be taken during product storage and support extrapolations over its shelf-life.
2.3. In-use stability study: For those drug products or cosmetics that have to be diluted or reconstituted prior to use, or those presented in a multi-dose container that, once opened is more sensitive to undergo alterations. Its purpose is to provide the information to be included in the labeling on the storage conditions and shelf-life of the preparation once reconstituted or opened. Stability is evaluated under the recommended conditions of use and during the proposed in use shelf-life.
3. Shortened stability studies: For existing active substances and related medicinal products, and for variations of an existing authorization, according to ICH specific guidelines and the ones developed by the EMEA or by the FDA.
|
Advantages |
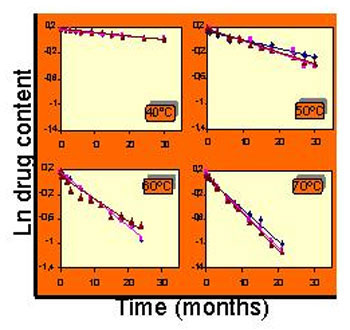
Stress stability study. Influence of temperature on the chemical stability of a product.
The technology allows a reduction of costs and time (equipment and personnel). Only by submitting the samples, you can obtain a detailed report of the study, carried out according to the corresponding regulations, suitable for incorporation in the registration dossier of a new medication or cosmetic; or an analysis of causes of instability and proposal of resources for stabilization, at the step of drug or cosmetic development.
|
Where has it been developed |
The research group responsible for this technology develops its work in the University Institute of Industrial Pharmacy, located in the Faculty of Pharmacy. The group has extensive research experience in this field, has published a book and numerous articles in international journals and has been invited to give international courses and conferences on this subject. The Institute has sufficient equipment to carry out stability studies (stability and photo stability cameras), as well as for the analysis of the physical properties of various pharmaceutical forms (solid, semi-solid and liquid) and chemical properties (HPLC, DSC, Spectrophotometer).
|
And also |
The group seeks public or private partners of the pharmaceutical, veterinary or cosmetic industry, of any size, national or international, who need to know the stability of their products and possible resources for their stabilization, or need to establish the shelf-life of a product in view of requesting the marketing authorization. The technique fits into pharmaceutical research and development departments of the above-mentioned industries.
|
Contact |
|
© Office for the Transfer of Research Results – UCM |
|
Classification |
|
Responsible Researcher |
Ana Isabel Torres Suárez: galaaaa@farm.ucm.es
Department: Pharmaceutics and Food Technology
Faculty: Pharmacy


