MICRONIZATION OF MATERIALS USING SUPERCRITICAL CO2 AS ANTISOLVENT AGENT
|
Description |
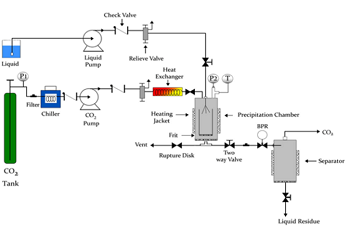
Scheme of a SAS device
A clean technology to micronize solvent-free materials using supercritical CO2 as an antisolvent is proposed. The method can be applied to the micronization of drugs, pigments, polymers, explosives, inorganic precursors, these compounds are generally large or polar molecules that exhibit low solubility in supercritical CO2, but they are very soluble in polar organic solvents. The technique is based on the antisolvent effect that supercritical CO2 has after being mixed with a solution of the compound in an organic solvent.
The SAS method allows controlling the morphology, size and/or polymorphic phase of the precipitated material, by varying the solvent and the pressure and temperature conditions (always moderate conditions) and, at the same time, it leads to solvent-free materials.
|
How does it work |
At temperatures above 31 ºC and pressures greater than 7.4 MPa, CO2 becomes a supercritical fluid. Supercritical CO2 has densities intermediate between those of liquids and gases, but transport properties (diffusivity and viscosity) similar to gases. When CO2 contacts the organic solution with the solute to be micronized and is dissolved in the solvent, the liquid experiences a volumetric expansion and becomes a bad solvent of the solute, which precipitates as micro and nanoparticles. The morphology, crystal structure and particle size can be controlled by modifying the temperature and pressure of the process, the solvent, etc… Solvent-free particles exhibiting a narrow particle size distribution are obtained.
A scheme of the experimental device used is shown in figure 1. A high pressure pump drives supercritical CO2 at the working temperature and pressure towards the precipitation chamber which is the same conditions at constant flow. The liquid solution formed by the solid and the organic solvent is also introduced at constant flow into the precipitation chamber through a nozzle. Current precipitation temperatures vary from 35 to 70 ºC, at pressures ranging from 8 to 20 MPa. When the supercritical fluid dissolves in the organic solvent, the solution becomes supersaturated and the solute precipitation starts. Pressure is controlled by a back pressure regulator at the exit of the precipitation chamber. The micronized solid is collected in the precipitation chamber whilst the CO2 + organic solvent mixture is separated in a cyclone separator. The precipitate is washed with supercritical CO2 to remove any solvent residue.
The micronized materials are characterized by X ray diffraction (low and wide angle), thermal analysis (TGA, DTA), FTIR and UV-vis spectroscopy, electron microscopy (SEM and TEM), composition analysis by EDX and microanalysis. Dissolution rate assays of the drugs are also performed.
Using this technique, different drugs such as diflunisal (DF- an analgesic and anti-inflammatory API), and different drug formulations such as those formed by DF and the polymer PVP, and the cocrystals formed by DF and nicotinamide (NIC- water soluble compound member of the vitamin B family). Figure 2 shows SEM images of these materials. In every case, due to the change of properties in the formulation and to the morphology and size control achieved using the SAS process, all the composites prepared show an improvement of the dissolution rate in comparison to the pure drugs.
|
Advantages |

Drugs precipitated by SAS
Traditional micronization techniques such as grinding, crystalization, “spray-drying” or “freeze drying” present many limitations such as the poor control of particle size and, sometimes, the high temperature required to remove the solvent. On the contrary, precipitation by SAS leads to solvent-free materials and allows controlling the morphology, size and polymorphic phase of the precipitated material.
CO2 is considered a green solvent because it has moderate critical parameters, it is cheap, non-toxic, inert, nonflammable and it can be recycled. Furthermore, CO2 is a gas at atmospheric pressure that can be easily released from the material by depressurization and it does not leave any residue.
|
Where has it been developed |
This technology has been developed at theLaboratory of Phase Equilibrium and Supercritical Fluids at the Faculty of Chemistry at UCM by the research group “Preparation and activity of multifunctional materials and physicochemical processes in Green Chemistry” which works in the preparation of materials using supercritical fluids as alternative to the common more contaminant organic solvents. Besides being sustainable processes, the materials obtained exhibit better properties tan those obtained by other conventional methods. The group has wide experience in the field of supercritical fluids both in fundamental and applied research.
|
And also |
The group belongs to the Spanish Royal Society of Chemistry (RSEQ), the Spanish Association of Compressed Fluids (FLUCOMP), the International Society for the Advancement of Supercritical Fluids (ISSASF) and the Green Chemistry Network.
We are interested in establishing collaborations with industry as well as with other research groups wishing to use this technique in those situations in which the conventional techniques do not yield satisfactory results, aiming to obtain high added value products. The group has a SAS precipitator based on the CO2 antisolvent effect. This equipment allows to perform micronization experiments at laboratory scale. Equipment to carry out the characterization of the materials produced is also available. We offer other technology knowledge transfer offers based in the use of supercritical CO2.
|
Contact |
|
© Office for the Transfer of Research Results – UCM |
|
PDF Downloads |
|
Classification |
|
Responsible Researchers |
Albertina Cabañas Poveda: a.cabanas@quim.ucm.es
Concepción Pando García-Pumarino: pando@quim.ucm.es
Department: Physical Chemistry
Faculty: Chemical Sciences


