MICROENCAPSULATION
|
Description |
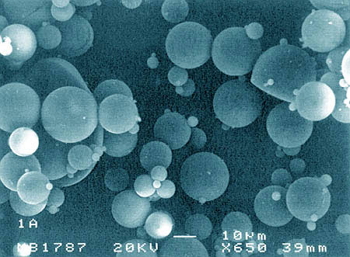
Spherical microparticles containing an antineoplastic agent to avoid its systemic toxicity.
Coating of powdery solid products or liquids with a film of polymeric or fatty material giving rise to free-flow particles of micron size. The substances to be encapsulated may be in the solid or liquid state. As a coating material different types of polymers can be used: natural (as alginate and chitosan), semisynthetic (as cellulose derivatives) or synthetic (aliphatic polyesters, polyorthoesters, polyalkylcyanoacrylates ...). These polymeric materials may be biodegradable (by the action of environmental agents or by enzymes or body fluids) or non-biodegradable (which may lead to systems with high persistence). Fatty substances with different melting points can also be used. Two types of structures can be obtained: reservoir type, in which the encapsulated substance, in the form of micrometric particles or liquid droplets is surrounded by the coating material forming an insulating cover from the outside; Or matrix type, wherein the encapsulated substance is dispersed, in the form of micrometric particles or at the molecular state, in a matrix of the coating material. The encapsulated substance may be released from the microparticles by degradation or melting of the coating, by mechanical rupture of the system or by slow diffusion through the structure. By suitable selection of the type of coating material and the structure of the microcapsules the rate of release of the encapsulated substance or the conditions under which it is produced can be modulated.
The micrometric size of the systems together with their versatility in terms of the release conditions of the encapsulated substance make microencapsulation a technology with numerous applications in different areas, such as drug development, cosmetics, food technology, plant protection area, biocides...
|
How does it work |
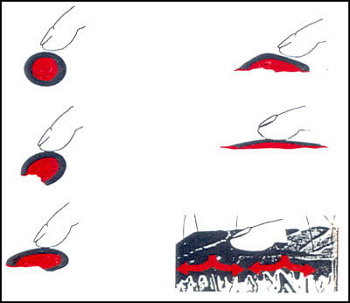
Application on skin of a microencapsulated cosmetic product.
There are different microencapsulation methods. The selection depends on:
The characteristics of the material to be encapsulated: if it is a solid or a liquid; its stability in different solvents; its stability against high temperatures, its chemical compatibility with the coating material.
The coating material used: its selection directly depends on the objective of the microencapsulation (see section “advantages”). Not all the coating materials can be applied by using any microencapsulation method.
The equipment available: the methods based on the formation of emulsions only need thermostatic containers provided of an agitation system, whereas the mechanical methods need more specific equipment (special centrifuges, extrusion nozzles, spray driers, fluid beds).
Essentially, the microencapsulation is based on the deposit of the coating material, in liquid state (due to its fusion or dissolution in a solvent), on the material to be encapsulated, which is dispersed as small particles (in case of a solid) o droplets (in case of a liquid) in an appropriated medium (liquid or gas). In a later step, the solidification of the coating is produced and microparticles are obtained. These microparticles will be collected, washed and conditioned in a different way depending on their application. Among the works carried out with this technology are:
- Microencapsulation of sodium butyrate by a variant of the solvent evaporation technique based on the formation of non-aqueous emulsions and using polymers of pH-dependent solubility in order to achieve a delayed release of the active substance at the intestine level after its oral administration.
- Microencapsulation of perfumes, using the complex coacervation technique with gelatin and gum arabic as polymers; for their application in adhesive strips which release the perfume after breaking the microcapsules.
- Microencapsulation of oils, using the complex coacervation technique with gelatin-gum arabic as polymers; and by the spray drying technique using different coating materials. The purpose of microencapsulation is to protect the active against oxidation, to avoid its unpleasant smell and taste and to transform it into a free-flowing powder product, easy to incorporate into different processed products.
- Microencapsulation of insecticides by means of the interfacial polymerization technique, obtaining a non-biodegradable polyurea coating that prevents the toxicity of the insecticide on mammals, birds and fish in an accidental ingestion or contact, by which the insects break with their jaws and ingest the insecticide.
- Microencapsulation of naloxone, by the technique of evaporation-solvent extraction using biodegradable polymers of the group of aliphatic polyesters. Matrix-type systems are available for subcutaneous administration, and release the active substance over time, resulting in an alternative to improve compliance in patients undergoing opiate withdrawal.
- Microencapsulation of dexamethasone, by means of the evaporation-solvent extraction technique using biodegradable polymers of the group of aliphatic polyesters; and by the spray drying technique using lipid coatings. Different types of parenteral administration systems are obtained in which the effect of glucocorticoid is prolonged and potentiated because of the increase of their interaction and internalization into cells.
- Microencapsulation of proteins by means of the evaporation-solvent extraction technique based on the formation of multiple emulsions and using biodegradable polymers of the group of aliphatic polyesters. When the systems are administered parenterally, a prolonged release of the active is achieved, avoiding its exposure to the tissue proteases that cause its degradation.
- Microencapsulation of cannabinoids by means of the evaporation-solvent extraction technique and using biodegradable polymers of the group of aliphatic polyesters. A free flow product is obtained, which solves the manipulation and dosing problems associated with the high lipophilicity of these compounds; providing prolonged release of the active substances for 2-4 weeks after subcutaneous administration, with a bioavailability significantly higher than those present orally or sublingually.
|
Advantages |
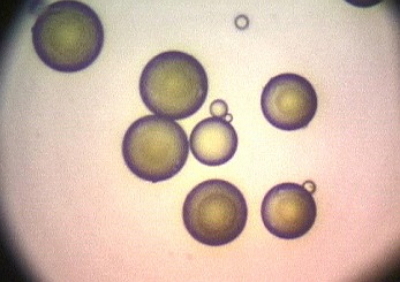
Microcapsules with an insecticide inside.
There are numerous reasons why it may be interesting to microencapsulate a drug. Some of them are: To avoid loss of volatile compounds (flavors in feeding, perfumes in cosmetic, drugs in pharmacy..), to mix incompatible compounds, to avoid degradation of the products due to environmental agents (in agriculture, pharmacy, cosmetic...), to prevent the irritant effect of certain drug substances when they are administered, to increase the duration of the effect of the drug substances due to a slow release after their administration.
|
Where has it been developed |
This technique has been developed in the University Institute of Industrial Pharmacy, located in the Faculty of Pharmacy. The Institute has all the necessary equipment to apply most of the microencapsulation techniques: reacting tanks, blade stirrers, propellers and turbines, homogenizers of different type, atomizer (Spray drier). Additional equipment for collection and conditioning of the resulting product (filtration equipment, centrifuges, drying cabinets, freeze drier), and for analysis and control thereof (HPLC, DSC, laser particle size analyzer, equipment for release studies, etc.)
|
And also |
- Microencapsulation of any type of materials.
- Advice on the advantages, technique and variables of the process
- Resolution of formulation problems.
- Analysis and control of microcapsules, including content release studies and stability studies
- Training of personnel: theoretical and practical teaching of the different techniques of microencapsulation.
|
Contact |
|
© Office for the Transfer of Research Results – UCM |
|
PDF Downloads |
|
Classification |
|
Responsible Researcher |
Ana Isabel Torres Suárez: galaaaa@farm.ucm.es
Department: Pharmaceutics and Food Technology
Faculty: Pharmacy


