EXTRACTION THROUGH SUPERCRYTIC CO2
|
Description |

Extractor of the Laboratory of Processes in Supercritical Fluids.
Supercritical fluids (SCFs) are particularly good solvents due to their ability to dissolve substances similarly to organic solvents, and because their viscosity and diffusion coefficient are close to those of the gases, thus facilitating the transport properties of these fluids. In addition, since the surface tension of the FSCs is zero, these fluids are particularly suitable for the extraction of substances contained in solid matrices. Another advantage in the use of FSCs is the possibility of changing their solvation power by variations in the pressure and / or temperature of the fluid, thus allowing the fractional extraction of the solutes, and the complete recovery of the solvent by simple adjustments of the pressure.
Of all the supercritical fluids studied, carbon dioxide (CO2) is the most used because of its low critical temperature (TC = 31ºC) and pressure (PC = 74 bar), non-toxicity, availability and low cost. It is a "green" solvent that is found in the atmosphere, in food and beverages and from which no minimum content needs to be set in the extracts, so it can be safely used. In fact it is considered a GRAS solvent.
Extraction from solid substrates by means of SCFs has been carried out on a commercial scale for more than two decades, mainly applied to the food industry, such as decaffeination of coffee beans and tea leaves or extraction of Bitter (α-acids) hops. In Spain, the extraction of 2,4,6-trichloroanisole from cork has been applied on a large scale since 2005.
In addition it is very investigated in these applications:
• Extraction from vegetal biomass (including residues) and microalgae of oils and fats, colorants, bioactive compounds, supplements (nutraceuticals) for food, animal nutrition and for pharmacy products, parapharmacy and cosmetics.
• Elimination of impurities such as insecticides from cereals, vegetables and soils; Organic oils from metal parts and electronic components; Residues of biomaterials fats and surgical material.
• Dry cleaning of fabrics and cultural material.
Given the high fixed costs, it is a suitable process for high productions and / or unit price of "relatively high" product. There is a high technological maturity and any application could be carried out on an industrial scale in a short period of time.
|
How does it work |
All processes of supercritical extraction (SCE) of solids consist of two stages: the extraction and the separation of the extract from the solvent. In the extraction, the supercritical CO2 flows through the solid and dissolves the extractable components. The solvent charged with the extract is evacuated from the extractor and fed to the separator where the pressure is reduced so that the solute is not soluble and precipitates. Alternatively, the solute can be separated from the supercritical solvent by adsorption, absorption or with a membrane. In this case, the solvent circuit can be operated at almost constant pressure.
Generally, a pre-treatment step of the solid material is required. Frequent addition of water to facilitate extraction. This amount is between 10 and 40%. Other times it is important to adjust the pH. If the extracted solute is the product of interest, the raw material may require milling to increase the extraction rate.
In general terms, the processes of extraction with supercritical CO2 are executed at temperatures of 35-40ºC, except in processes where the solute is strongly adsorbed. In such cases, higher temperatures are required to aid desorption (up to 70-80 ° C). The pressure ranges from 100 to 500 bar, depending on the type of extract.
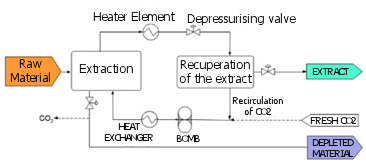
Schematic of an extraction process with supercritical CO2 with extract recovery by reduction in pressure.
|
Advantages |
1. After the extraction, the CO2 can be removed in gaseous form without leaving any remains in the matrix or in the solute;
2. The aroma and flavor of the extracts obtained is more natural;
3. The degradation of the product is minimized and the extractions are faster and more efficient since the selectivity is very high. Hence, the purification process of the extract is very simple or even not necessary, versus the conventional extractions with organic solvents that require long and expensive processes of concentration and purification.
4. The solvent is recovered and recirculated easily. Therefore, the supercritical extraction can be considered a sustainable and intensive process.
|
Where has it been developed |
The Supercritical Fluid Processes group of the Department of Chemical Engineering has extensive experience in technology. Some of the processes that have investigated are:
Extraction of:
- Vegetable oils
-Seeds.
-Microalgae.
- Cocoa butter
- Paprika oleoresin.
- Caffeine from coffee residues.
- Cork trichloroanisole or cereal pesticides.
Cleaning of:
- Metallic contacts (of lubricating and mechanical oils).
|
And also |
• Sterilization of sensitive materials using supercritical CO2
• Formation of nanoparticles by supercritical extraction of emulsions
|
Contact |
|
© Office for the Transfer of Research Results – UCM |
|
PDF Downloads |
|
Classification |
|
Responsible Researcher |
Lourdes Calvo Garrido: lcalvo@ucm.es
Department: Chemical Engineering
Faculty: Chemical Sciences


