ENZYMATIC BIOTECHNOLOGY AND RELATED INDUSTRIAL BIOTRANSFORMATIONS
|
Description |
Enzymes, as biological catalysts, show very interesting properties for the industry: they are highly specific, work in mild conditions, are easily available and preserve the environment. For these reasons, many enzyme-catalysed processes have emerged in food, detergent, chemical, pharmaceutical and diagnostic industry. These applications have demonstrated to be very competitive and efficient. Our technology offers the possibility to perform a biotransformation which leads to the product that the customer needs.
|
How does it work |
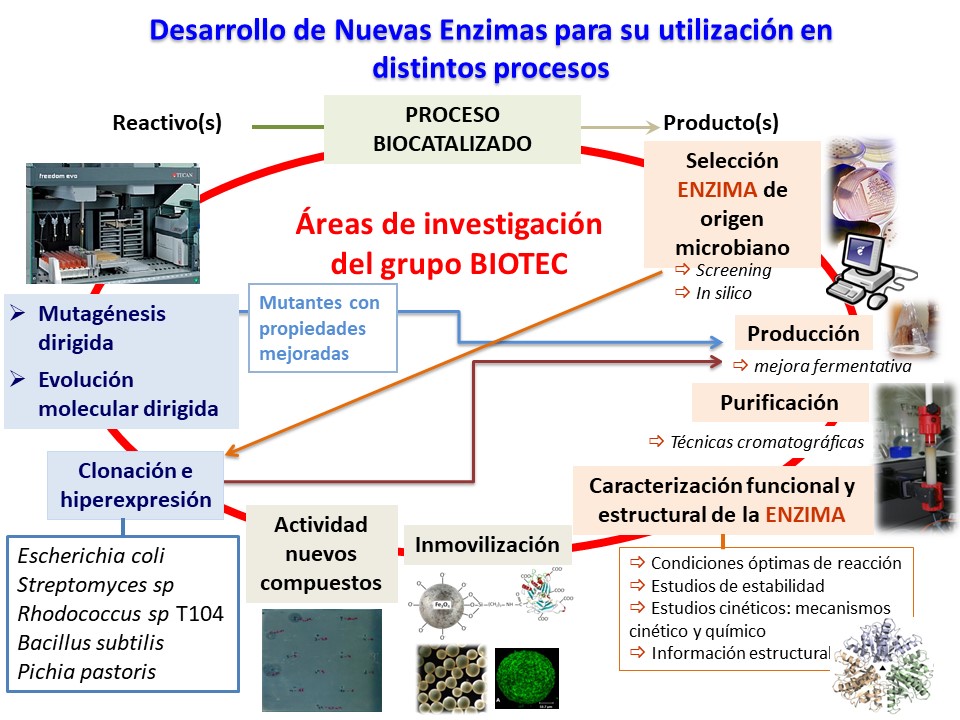
BIOTEC group new enzyme development platform.
Enzymes are obtained from microorganisms (bacteria, fungi or yeasts) selected by screening and harvested by fermentation procedures (reactors or flasks). Enzyme purification is achieved from the fermentation broth to yield the biocatalyst of commercial interest. The purification procedure involves any chromatographic technique and concentration techniques are utilized.
Structural characterization of the purified enzyme is performed by electrophoretic techniques, MALDI-TOF, analytic ultracentrifugation and spectroscopy studies, as well as functional characterization, substrate specificity, determination of kinetical and chemical mechanisms and stability in the working conditions. These data allow to carry on site-directed mutagenesis to obtain new enzymes to be used in industrial bioreactors.
Isolation, cloning and sequencing of the gene which codifies for the synthesis of the enzyme allow to obtain higher amounts of protein as well as carrying studies of protein engineering. If both cloning and overexpression in by E. coli fails because of peculiarities of the genome or necessity from posttranslational processes, other systems of clonation and expression are utilized. Genes from actinomycetes will be cloned and expressed in Streptomyces lividans by using bifunctional expression vectors. Another cloning and expression system would be Pichia pastoris.
Wild type or cloned enzymes and their mutants with better properties, are immobilized to allow their recovery and recycling in the process. Different immobilization methods adapted to the industrial process are used: gel entrapment, microencapsulation, cross-linking with bifunctional reactants, adsorption and covalent linkage (inorganic and organic supports). Once the method has been chose, the optimisation of the immobilization process is studied in order to achieve an industrial biocatalyst. Finally, best conditions for process are determined: optimal pH value and temperature, pH and temperature stability, determination of kinetic parameters and recycling.
|
Advantages |
Through systems of developed expressions, any enzyme of industrial and/or pharmacological interest can be obtained in large quantities, bringing down processes costs. The novelty lies in the disposition of non-conventional systems of clonation and expression, which allow producing enzymes and other molecules that cannot be obtained using E. coli, like in the case of the ones produced by actinobacteria, microorganisms’ source of a great variety of compounds for industrial and/or pharmacological interest.
Immobilization is a technology that allows the acquisition of enzymatic derivatives more stable than the enzyme in solution, and that can be reused several times. It also allows the design of reactors of easy manage and control.
The novelty is the use of activated supports allowing multipoint covalent immobilization of enzymes, representing a significant increase in stability and maintenance of the activity after successive cycles since its desorption occurs.
Enzymes are very specific biocatalysts, exhibit high catalytic activity and act at moderate temperatures and atmospheric pressure. In addition, reactions in organic media have numerous advantages over aqueous media: increase the solubility of some reactants, products can be isolated more easily, enzyme stability may be increased, no bacterial contamination, etc.
The ability to produce enzymes in large quantities and dispose of them in a stable and reusable way would reduce production costs of any process, using these biocatalysts would not generate polluting chemicals that often accompany chemical processes. This not only cheapen production costs by not having to apply methods of decontamination, but also the process itself involves an environmental improvement.
|
Where has it been developed |
This technology is developed in the Department of Biochemistry and Molecular Biology I, Faculty of Biological Sciences. The team has extensive experience in the methodologies described and has worked with different enzymes (cellulases, lipases D-amino acid oxidase, penicillin acylases) for industrial application.
The research group aims enzymes involved in industrial processes.
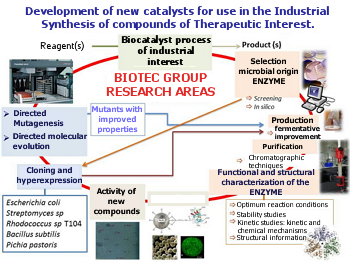
Research areas of BIOTEC group.
|
And also |
We are looking food, detergent, chemical products, pharmaceuticals and diagnostic companies who desire to obtain great quantitites of a particular biocatalizer in enzymatic biorreactors, in a process of low economic cost. The Enzymatic Biotechnology Group from the Complutense University of Madrid offers his know how to optimise industrial enzymes: production, isolation, purification and characterization from producer microorganisms; isolation, cloning and sequencing of the genes; hyperexpression in microorganisms; protein engineering, immobilization and optimisation to apply the enzymes in bioreactors. The group can produce any enzyme for companies interested in obtaining industrial biocatalysts for any application field.
|
Contact |
|
© Office for the Transfer of Research Results – UCM |
|
PDF Downloads |
|
Classification |
|
Responsible Researcher |
Isabel de la Mata: idlmata@ucm.es
Department: Biochemistry and Molecular Biology I
Faculty: Biological Sciences


