DEPOSITION OF SUPPORTED METAL AND METAL OXIDE NANOPARTICLES USING SUPERCRITICAL CO2
|
Description |
A clean technology to deposit metal and metal oxide nanoparticles within porous and planar substrates with complex structures using supercritical CO2 is proposed.
Deposition of metal and metal oxides on porous supports has a great interest due to the large number of applications of these composite materials. They are used in heterogeneous catalysis, fuel cells, medicine (contrast agents or controlled drug release), as sensors, to strengthen fibers, materials for H2 storage, in microelectronics…
The porous support stabilizes the nanoparticles, inhibits their growth and aggregation and, at the same time, it preserves the properties of the nanoparticles. On the other hand, nanoparticles deposited on the support create active sites in the material and can improve the properties of the supports and nanoparticles themselves.
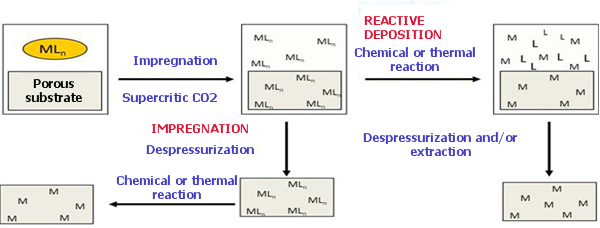
Scheme of the method of material deposition using supercritic CO2
|
How does it work |
At temperatures above 31 ºC and pressures greater than 7.4 MPa, CO2 becomes a supercritical fluid. Supercritical CO2 has densities intermediate between those of liquids and gases, but transport properties (diffusivity and viscosity) similar to gases. All these properties and the low surface tension of CO2 with solid surfaces allow to introduce metal precursors dissolved in CO2 inside porous materials in a very efficient way. Furthermore, the properties of CO2 can be tuned through small changes of pressure and temperature, changing the final properties of the material.
A scheme of the procedure is given in Figure 1. The metal precursor dissolved in the supercritical CO2 impregnates the support, and it is then chemically or thermally decomposed. The decomposition can be carried out after the depressurization or at supercritical conditions. If the decomposition is carried out after depressurization, metal nanoparticles are homogeneously dispersed within the support; if the reduction is carried out at supercritical conditions larger particles, nanowires or continuous films are obtained.

High pressure stirred reactor.
Using this method, we have already deposited Pd, Pt, Ru and Ni nanoparticles and several metal oxides on ordered mesoporous silica, mesoporous carbons and reduced graphene oxide. Experiments were performed using stirred high-pressure reactors (figure 2). The high catalytic activity and selectivity of the Pd, Pt and Ru supported materials was shown in model hydrogenation reactions.
Structural material characterization of the materials was carried out by X-ray diffraction (low and wide angle), thermal analysis (TGA, DTA), N2-adsorption, FTIR and UV-vis spectroscopy, electron microscopy (SEM and TEM), composition analysis by EDX and ICP-OES.
|
Advantages |
Using supercritical CO2 as solvent and reaction medium in the synthesis of the composite materials, metal precursors can be introduced within the micro and mesopores of different substrates in a more efficient way than with conventional methods (in liquid or gas phase). The materials produced are very homogeneous at microscopic level and nanoparticles present a regular shape and size with a narrow particle size distribution and, at the same time, they are homogeneously dispersed throughout the materials (figure 3). The impregnation process in CO2 maintains intact the structure of the support.
CO2 is considered a Green solvent because it has moderate critical parameters, it is cheap, non-toxic, inert, nonflammable and it can be recycled. Furthermore, CO2 is a gas at atmospheric pressure that can be easily released from the material by depressurization and it does not leave any residue.
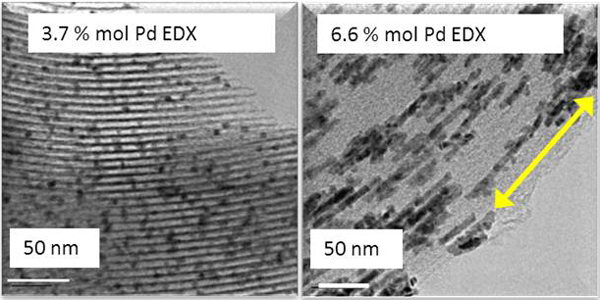
Nanoparticles and Pd nanowires deposited inside the mesopores of SiO2 SBA-15 using supercritical CO2.
|
Where has it been developed |
This technology has been developed at the Laboratory of Phase Equilibrium and Supercritical Fluids at the Faculty of Chemistry at UCM by the research group “Preparation and activity of multifunctional materials and physicochemical processes in Green Chemistry” which works in the preparation of materials using supercritical fluids as alternative to the common more contaminant organic solvents. Besides being sustainable processes, the materials obtained exhibit better properties tan those obtained by other more conventional methods. The group has wide experience in the field of supercritical fluids both in fundamental and applied research and presents several technology offers.
|
And also |
The group belongs to the Spanish Royal Society of Chemistry (RSEQ), the Spanish Association of Compressed Fluids (FLUCOMP), the International Society for the Advancement of Supercritical Fluids (ISSASF) and the Green Chemistry Network.
We are interested in establishing collaborations with Industry as well as with other research groups wishing to use this technique in those situations in which the conventional techniques do not yield satisfactory results, aiming to obtain high added value products. The group has reactors and the high pressure equipment required to carry out the experiments at laboratory scale, as well as equipment to carry out the characterization of the materials produced.
|
Contact |
|
© Office for the Transfer of Research Results – UCM |
|
PDF Downloads |
|
Classification |
|
Responsible Researcher |
Albertina Cabañas Poveda: a.cabanas@quim.ucm.es
Departament: Physical-Chemistry I
Faculty: Chemical Sciences


