ATOMIZATION OF NASAL POWDER DRUG FORMULATIONS BY SPRAY-DRYING
|
Description |
Figure 1. Scheme of the atomization process.
Spray-drying is a procedure widely used by different industries because is a continuous process of production which allows a fast and non-degradable way to dry many products. Recently, in the Department of Pharmaceutical Technology of the Complutense University of Madrid a new procedure to obtain spray-dried powders has been developed. Spray-dried powders obtained by this new technique have been proved to be useful as nasal absorption enhancers.
|
How does it work |

Figure 2. Micrograph of the nasal powder.
It is well known that insoluble excipients with high water absorption ability can enhance nasal absorption of drugs because they can modify tensions in the cytoskeleton or tight junction proteins. In order to obtain such an effect an intimate union between drug and excipient is required because physical pattern deposition of the drug must be the same than the excipient.
A simple physical mixture of drug and excipient does not guarantee such an intimate union. Meanwhile, the usual freeze-drying process that is a suitable method to obtain this effect is a long and expensive industrial process. Conventional spray-drying of drug and excipient in a liquid medium in which drug is solubilized and excipient is in suspension leads to the production of two different types of particles (see figure 2). Some of them will correspond to the excipient with part of drugs (in our example large and fibrous particles) and others will be of pure drug (small spherical particles in figure 2).
As these two different types of particles present very different size and shape they should have different deposition patterns in the nasal cavity and, therefore, drug absorption cannot be efficiently enhance because this effect is limited to the vicinity of the excipient particles responsible for tight-junction opening. To avoid the production of these two populations of particles a new method is proposed. In a first stage the drug has been incorporated into the excipient as a solution, taking advantage of the water intake ability of the excipient. Then the excipient particles are dispersed in a liquid medium in which neither the drug nor the excipient are soluble and then spray-dried. The resultant powder is an intimate union of drug and excipient suitable for nasal administration (see figure 3).
|
Advantages |
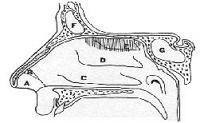
Figure 3. Diagram of the nasal cavity.
Spray-drying is a faster and cheaper process than freeze-drying. It is suitable for labile products as peptides and proteins.
|
Where has it been developed |
The department of Galenic Pharmacy and Food Technology of the Complutense University of Madrid has an extended experience on drug formulation collaboration with many Pharmaceutical Companies. In this department this new procedure has been developed and then, the Complutense University has patented it. This method has been proved to be suitable for a successfully in vivo nasal administration of cyanocobalamin although it can also be applied to other type of drugs. The clinical administration of nasal powders is well documented and it is frequently used for peptide drugs, as can be calcitonin.
|
And also |
The group has drug development experience in oral, topical and parenteral administration.
|
Contact |
|
© Office for the Transfer of Research Results – UCM |
|
PDF Downloads |
|
Classification |
|
Responsible Researcher |
Juan. J Torrado Durán: torrado1@farm.ucm.es
Department: Galenic Pharmacy and Food Technology
Faculty: Pharmacy


