ASSESSMENT OF ANALGESIC AND ANTI-INFLAMMATORY EFFICACY IN THIRD LOWER MOLAR SURGERY
|
Description |
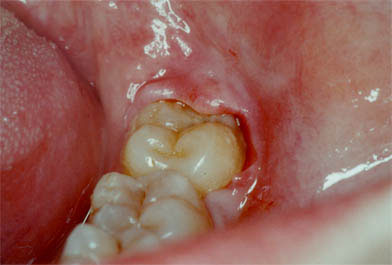
Third lower molar with infectious manifestation in the form of pericoronaritis that will require surgical treatment
Clinical trials to determine, through comparative studies, the efficacy of antibiotic, analgesic and anti-inflammatory drugs in the model of lower third molar surgery where administration of antibiotics, analgesics and anti-inflammatories is common.
|
How does it work |
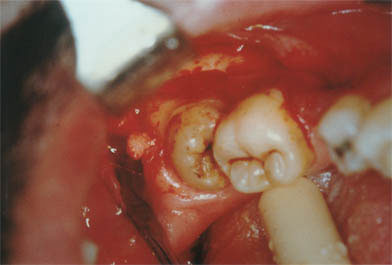
Surgical phase in which ostectomy is performed for the release of the lower third molar
Randomized patient groups undergoing third molar surgery are used. After surgery, once they reach type 2 pain in semiquantitative scales, the patients ingest the product and perform controls at 30 minutes, one, two, three, four, five and six hours in which the patient records on semiquantitative scales and visual analog scales, intensity and relief they may have had. In the case of antibiotics, culture samples are taken before the intervention, at 48 hours and seven days, and the colony count and identification of the colonies are evaluated.
|
Advantages |
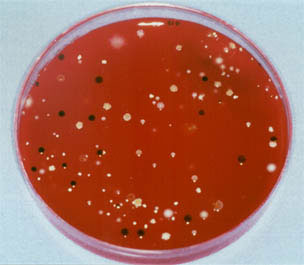
Growth of colonies taken at 48 hours of treatment of a lower third molar
It is a model of experimentation universally accepted for this type of study, being also simple and fast to obtain.
|
Where has it been developed |
The studies are done in the Department of Stomatology III (Medicine and Bucofacial Surgery) of the Complutense University of Madrid.
|
And also |
Studies of different drugs in development phase or marketed by Pharmaceutical Laboratories, being able in addition:
• Adapt the technology to the specific problems of the client.
• Conduct technical feasibility studies for a specific application.
• Training for the use of the technology in question, etc.
|
Contact |
|
© Office for the Transfer of Research Results – UCM |
|
PDF Downloads |
|
Classification |
|
Responsible Researcher |
José María Martínez González: jmargo@odon.ucm.es
Department: Stomatology III (Medicine and Oral Surgery)
Faculty: Dentistry


