ANIMAL MODEL TO IDENTIFY THERAPEUTICALLY USEFUL COMPOUNDS FOR THE SPORADIC AMYOTROPHIC LATERAL SCLEROSIS TREATMENT
|
Description |
A research group at the Complutense University of Madrid (UCM), together with researchers from the National Research Council (CSIC), have established an animal model that reproduces the symptoms of sporadic Amyotrophic Lateral Sclerosis (ALS), so that the disease appears and develops slowly. Therefore, this model represent a new tool to study the effectiveness of potentially therapeutic molecules with possibility of setting up effective treatments against sporadic ALS. Further, UCM and CSIC have achieved a patent to protect the new animal model and the method of analysis of the effectiveness of drugs, and look for companies interested in acquiring the rights to exploit the patent.
|
How does it work |
The new animal model developed by the UCM and CSIC is based in rats. These rats are treated with a neurotoxic amino acid, β-N-methylamino-L-alanine (L-BMAA) following doses and guidelines that have been established to reproduce the symptoms of ALS.
In its free form, the L-BMAA and carbamylated adduct can mediate neuronal degeneration regulated by activation of excitatory amino acid receptors mechanism. The β-carbamate form of L-BMAA is similar to glutamate and interacts with its cellular receptors. It is proposed that the mechanism of neurotoxicity of L-BMAA is based on three aspects: 1) direct action on NMDA, 2) activation of metabotropic glutamate receptors (mGluR5 and mGluR1) and 3) the induction of stress oxidative.
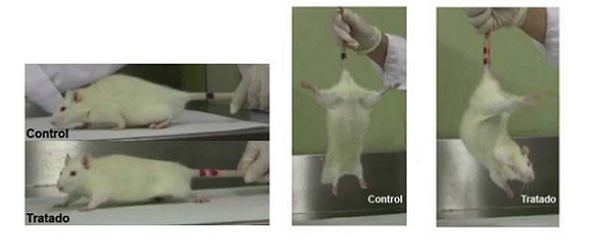
Test of force and test of suspension by the tail
To determine the affectation level of the L-BMAA treated animals, we have developed a scale ofneurological evaluation, based on ambulation, tail suspension test and strength test.
We have verified the validity of the model using different tests:
• Electronic microscopy studies which confirmed the alteration of motor neurons. These alterations have been observed in animals treated with L-BMAA, coinciding with signs of ALS patients;
• Biochemical analyzes have shown high levels of both GSK3 as high molecular weight forms and fragments truncated of TDP-43 in central nervous system of animals treated with L-BMAA;
• Tests conducted by HRMAS (High Resolution Magic Angle) with which it has been possible to detect and quantify neurodegeneration through the analysis of certain metabolites in the brain parenchyma (metabolites whose study has been previously used in trials in ALS patients).
The only animal model of ALS that exists nowadays is based on deficient rats in a gene involved in familial ALS. These rats undergo rapid appearance of symptoms, reaching an irreversible state in very short time, making it difficult to test potentially therapeutic molecules. Instead, in the new model, the symptoms appear gradually and slowly, allow testing potentially therapeutic active throughout the course of the disease and test whether reverse or slow down the symptoms.
ALS patients can choose for a number of drugs to combat the symptoms that accompany the disease, but there are still no drugs with ability to slow or reverse the symptoms caused by the disease. Hence the importance of the new animal model of ALS, which facilitates the study of active substances, could provide therapeutic tools against this disease.
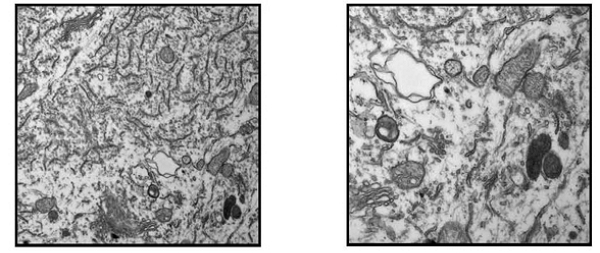
The images correspond to motoneurons of the spinal cord of treated animals, which exhibit endoplasmic reticulum fragmentation and mitochondrial disorganization
|
Advantages |
The model presented here is the only animal model for sporadic ALS, a variety that affects more than 90% of all patients with this disease. The main difference between animals treated with L-BMAA and the animal model of familial ALS is that the symptoms progress slowly, so you can see precisely the evolution of these symptoms and the effect of possible therapies.
Include two other aspects:
- Reproducibility of model: similar results were obtained in at least of the 90% of the animals treated with L-BMAA;
- Easy to be performed in vivo assays in rats.
|
Where hast it been developed |
The UCM group that develops this research is directed by Begoña Gómez Miguel. It is a multidisciplinary group that began in 2009 with the aim of carry out a model of sporadic amyotrophic lateral sclerosis in rats. The components of the group belong to the Faculty of Biological Sciences of UCM and the Institute of Medical Chemistry of the CSIC. All team members have extensive research experience and add the necessary skills to have developed this animal model and to test different potentially therapeutic molecules.
|
And also |
To protect the animal model and method to use, we have a patent in the Spanish Patent and Trademark Office which is still within the period in which you can apply for international expansion through a PCT application.
UCM and CSIC are interested in contacting companies who wish to acquire an operating license to exploit this patent.
|
Contact |
|
© Office for the Transfer of Research Results – UCM |
|
PDF Downloads |
|
Classification |
|
Responsible Researcher |
Begoña Gómez Miguel: bgomezmi@bio.ucm.es
Department: Biochemistry and Molecular Biology I
Faculty: Biological Sciences


