Research lines
Immunological synapse
The immunological synapse (IS) is the specialized adhesion where the molecular basis for leukocyte communication is organised. The IS contains three supramolecular activation clusters (SMAC) in central (c), peripheral (p), and distal (d) location (Figure 1) (1-10). Lymphocyte decisions are dictated by the molecular dynamics of signalling networks locally organized at the IS, which integrates signals from different surface receptors(4, 11, 12). We have particular interest on molecular dynamics during the IS established by NK cells and T cells with antigen presenting cells (Figure 2).
Figure 1: a) Three-dimensional confocal microscopy reveals the topography of the IS. Location of key molecules for lymphocyte activation in the different SMAC is colour coded. b) As an example, the distribution of the activating receptor 2B4 and talin at the cytotoxic NK cell IS is shown. Surface of the IS (dashed red line) is reconstructed from confocal sections as represented in a.
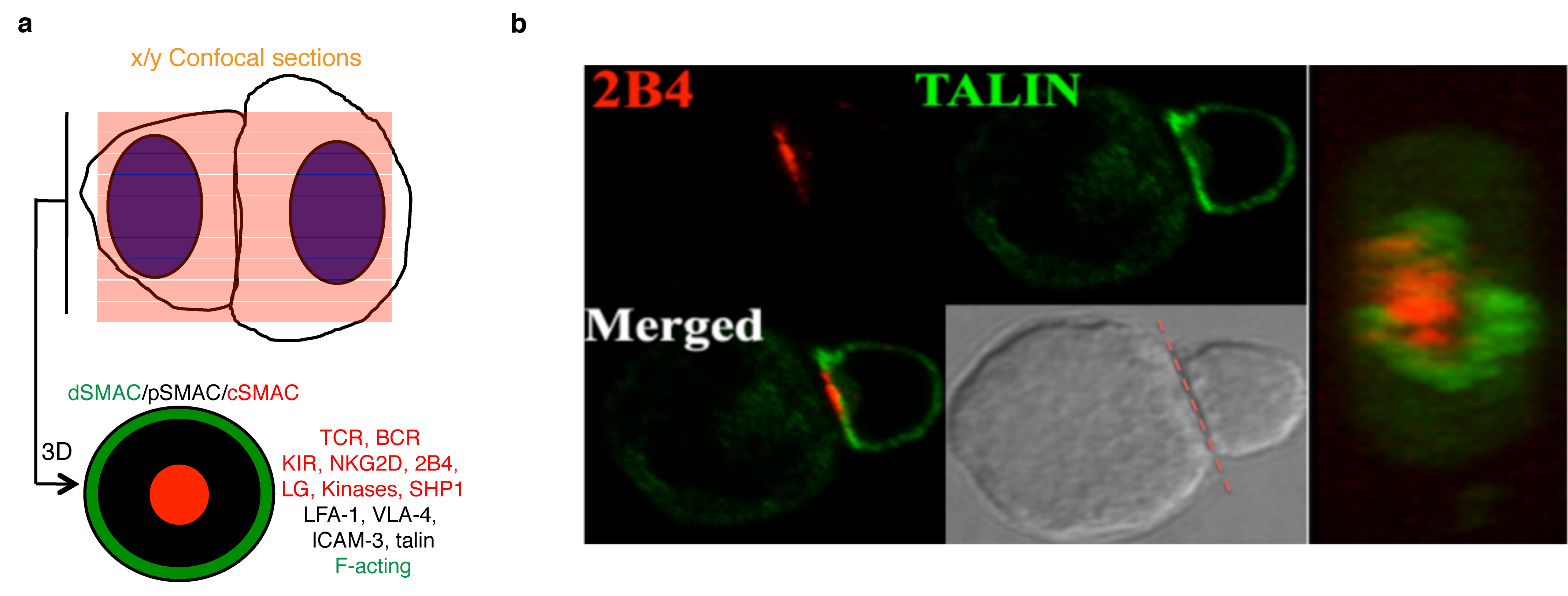
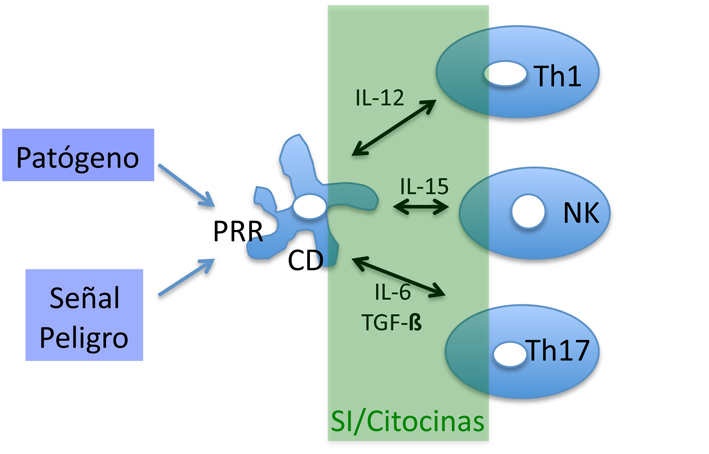
Figure 2: Dendritic cells (DC) promote T cell differentiation and NK cell activation upon pattern recognition receptor (PRR) stimulation. Green area underscores the action of IS and cytokines.
Polarised cytokine signalling at the activating NK cell IS.
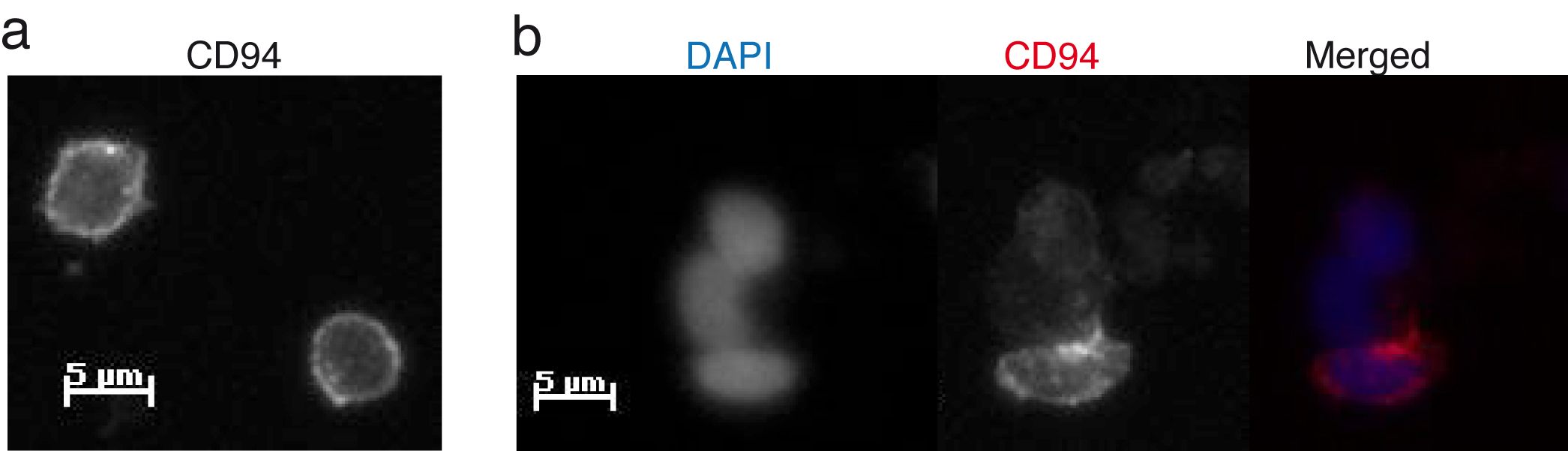
NK cell activation is enhanced by IL-12 or TNF-alfa produced by DC. In turn, IFN-gamma produced by activated NK cells enhances DC ability to produce these pro-inflammatory cytokines. This NK cell/DC crosstalk is mediated by the IS assembled following NK cell and DC adhesion, so called activating (a) or regulatory NK cell IS (aNK-IS)(6, 13, 14). This reciprocal regulation is mediated by the selective delivery into the aNK-IS of cytokines, including IL-12(15) and IL-18(16). Recruitment of the IL-15 receptor alfa chain (IL-15-Ralfa) to the aNK-IS induces activating and survival signals in NK cells(17). However, dynamics of polarised signalling at this IS has yet to be studied. Previously we have shown that patients with Rheumatoid Arthritis (RA) showed that IL-15 bound to the surface of monocytes (Mo) enhances IFN-gamma production by NK cells that, in turn, increases TNF-alfa production by Mo(18). Thus we think that local IL-15 signalling at the aNK-IS may promote inflammatory diseases like RA. To study this local IL-15 signalling at the IS, we differentiate DC from human peripheral blood monocytes, madurate them by TLR stimulation, and put along with ex vivo isolated NK cells to form the aNK-IS (Figure 3). We expect that this in vitro model will allow studying the local IL-15 signalling essential for NK cell activation.
Figure 3: aNK-IS. a) CD94 is evenly distributed on the surface of NK cells. b) Recruitment of CD94 reveals the formation of the aNK-IS(6).
Polarised signalling during T cell activation.
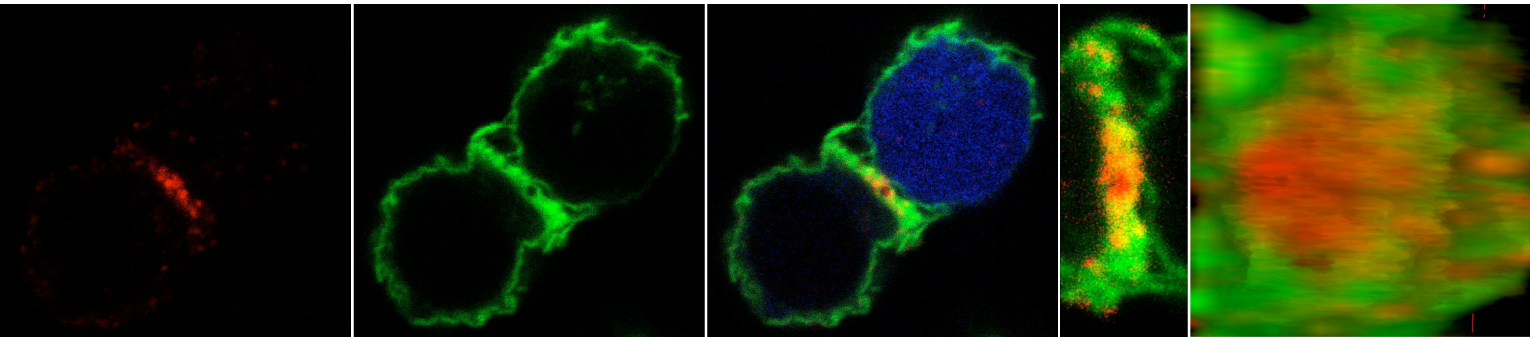
The molecular dynamics of polarised signalling at the IS appears to be essential for T cell activation/differentiation, and clonal expansion(11, 12, 19-22). For example, during activation of mouse naïve T cells by DC, IFN-gamma receptor and STAT1 are polarized to the IS. This constitutes a determinant of Th1 differentiation, which is inhibited by IL-4 and consequent Th2 differentiation(11, 12). Whether this sort of dynamic mechanism for T cell differentiation exists in human naïve lymphocytes is still not known(4). To address this issue we work in the laboratory with in vitro experimental models suitable for studying the IS based on the interaction of immortalised T cell lines or primary human T cells with antigen presenting cells (Figure 4).
Figure 4: T cell immune synapse. Confocal microscopy shows the accumulation of CD3 at the cSMAC (red) and the redistribution of filamentous (F) actin at the dSMAC (green). The topography of the T cell immune synapse obtained by 3D-CM is shown. The CD4+ T cell line Jurkat and the B cell line Raji loaded with Staphylococcal Enterotoxin E (SEE) as antigen presenting cell are used. Raji cells are labelled with Chloromethylcoumarin (CMAC) (blue).
Signalling networks; role of protein tyrosine phosphatases in lymphocyte activation
Protein tyrosine phosphatases (PTP) constitute a family of related enzymes encoded by 107 genes. Complexity of this family is increased by alternative processing of mRNA that generate a large number of isoforms(23). PTP can be classified as classical, specific for phospho-Tyr residues, and dual-specificity phosphatases that dephosphorylate phospho-Tyr/-Ser/-Thr residues, phosphoinositides and nucleic acids. The majority of cell types express between 30% and 60% of PTP-encoding genes. This percentage is nonetheless higher in lymphocytes, which express 60 to 70 PTP genes(24, 25). Thus we think that it is interesting to study their expression profiles in health and disease.
PTP activity and accessibility to substrates are regulated by posttranslational modifications, such as phosphorylation and oxidation, as well as conformational changes. PTP are multimodular proteins with domains that regulate their accessibility to substrates and intracellular signalling-competent sites(26). PTP activity is thus restricted in space and time(27, 28). In this regard, the relevance of PTP dynamics at the IS has been suggested. For example, antigenic stimulation promotes MAPK activation, and recruits the haematopoietic (He)PTP, a MAPK inhibitor, to the IS(29). This mechanism could assure transient activation of Erk, frequently needed for cell proliferation(30). We employ microspectroscopy techniques(10), to further studied PTP intracellular dynamics during IS establishment.
We are interested in still unresolved questions about this family of enzymes:
- The relevance of PTP dynamics for both, IS assembly and lymphocyte activation/differentiation, an issue poorly understood for the majority of PTP. We are interested in T cell IS and aNK-IS
- Abnormalities in dynamic regulatory mechanisms of PTP associated to autoimmune diseases, currently mostly unknown. A known example is the increased MAPK signalling associated to a delayed accumulation of SHP1 at the IS in lymphocytes of RA patients(31).
- Although phosphorylation is a posttranslational modification that regulates function and stability of SOCS, we do not know which PTP regulate their phosphorylation levels, and when and where this regulation takes place. This may be relevant for the local cytokine signalling at the IS(11, 12, 15, 17, 19, 20).
- PTP expression profiles associated to autoimmune diseases. We expect that this studies will provide information about gene alterations, such as polymorphisms, associated to these diseases, and therefore new potential PTP targets for therapy.
References
1. Monks CR, Freiberg BA, Kupfer H, Sciaky N, & Kupfer A (1998) Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395(6697):82-86.
2. Batista FD, Iber D, & Neuberger MS (2001) B cells acquire antigen from target cells after synapse formation. Nature 411(6836):489-494.
3. Stinchcombe JC, Bossi G, Booth S, & Griffiths GM (2001) The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15(5):751-761.
4. Egen JG & Allison JP (2002) Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity 16(1):23-35.
5. Montoya MC, Sancho D, Vicente-Manzanares M, & Sanchez-Madrid F (2002) Cell adhesion and polarity during immune interactions. Immunol Rev 186:68-82.
6. Roda-Navarro P (2009) Assembly and function of the natural killer cell immune synapse. Front Biosci 14:621-633.
7. Dustin ML & Depoil D (2011) New insights into the T cell synapse from single molecule techniques. Nat Rev Immunol 11(10):672-684.
8. Goodridge HS, et al. (2011) Activation of the innate immune receptor Dectin-1 upon formation of a 'phagocytic synapse'. Nature 472(7344):471-475.
9. Alarcon B, Mestre D, & Martinez-Martin N (2011) The immunological synapse: a cause or consequence of T-cell receptor triggering? Immunology 133(4):420-425.
10. Roda-Navarro P (2013) Microspectroscopy reveals mechanisms of lymphocyte activation. Integrative biology : quantitative biosciences from nano to macro 5(2):300-311.
11. Maldonado RA, Irvine DJ, Schreiber R, & Glimcher LH (2004) A role for the immunological synapse in lineage commitment of CD4 lymphocytes. Nature 431(7008):527-532.
12. Maldonado RA, et al. (2009) Control of T helper cell differentiation through cytokine receptor inclusion in the immunological synapse. J Exp Med 206(4):877-892.
13. Walzer T, Dalod M, Vivier E, & Zitvogel L (2005) Natural killer cell-dendritic cell crosstalk in the initiation of immune responses. Expert Opin Biol Ther 5 Suppl 1:S49-59.
14. Kalinski P, et al. (2005) Natural killer-dendritic cell cross-talk in cancer immunotherapy. Expert Opin Biol Ther 5(10):1303-1315.
15. Borg C, et al. (2004) NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood 104(10):3267-3275.
16. Semino C, Angelini G, Poggi A, & Rubartelli A (2005) NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood 106(2):609-616.
17. Brilot F, Strowig T, Roberts SM, Arrey F, & Munz C (2007) NK cell survival mediated through the regulatory synapse with human DCs requires IL-15Ralpha. J Clin Invest 117(11):3316-3329.
18. Gonzalez-Alvaro I, et al. (2006) Interleukin-15 and interferon-gamma participate in the cross-talk between natural killer and monocytic cells required for tumour necrosis factor production. Arthritis Res Ther 8(4):R88.
19. Huse M, Lillemeier BF, Kuhns MS, Chen DS, & Davis MM (2006) T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol 7(3):247-255.
20. Sabatos CA, et al. (2008) A synaptic basis for paracrine interleukin-2 signaling during homotypic T cell interaction. Immunity 29(2):238-248.
21. Grakoui A, et al. (1999) The immunological synapse: a molecular machine controlling T cell activation. Science 285(5425):221-227.
22. Lee KH, et al. (2002) T cell receptor signaling precedes immunological synapse formation. Science 295(5559):1539-1542.
23. Alonso A, et al. (2004) Protein tyrosine phosphatases in the human genome. Cell 117(6):699-711.
24. Mustelin T, Vang T, & Bottini N (2005) Protein tyrosine phosphatases and the immune response. Nat Rev Immunol 5(1):43-57.
25. Vang T, et al. (2008) Protein tyrosine phosphatases in autoimmunity. Annu Rev Immunol 26:29-55.
26. Tonks NK (2006) Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol 7(11):833-846.
27. Ostman A & Bohmer FD (2001) Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatases. Trends Cell Biol 11(6):258-266.
28. Tonks NK & Neel BG (2001) Combinatorial control of the specificity of protein tyrosine phosphatases. Curr Opin Cell Biol 13(2):182-195.
29. Nika K, et al. (2006) Lipid raft targeting of hematopoietic protein tyrosine phosphatase by protein kinase C theta-mediated phosphorylation. Mol Cell Biol 26(5):1806-1816.
30. Santos SD, Verveer PJ, & Bastiaens PI (2007) Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat Cell Biol 9(3):324-330.
31. Singh K, et al. (2009) ERK-dependent T cell receptor threshold calibration in rheumatoid arthritis. J Immunol 183(12):8258-8267.
