Photovoltaics
Organic Photovoltaics
All Organic Photovoltaic Devices
The implementation of organic photovoltaics as a clean and reliable energy source requires the availability of efficient, cheap, and flexible devices which, mimicking natural processes such as photosynthesis, are able to transform sunlight into power.
The foremost feature of organic photovoltaics is its flexibility (Figure 1) which represents their major advantage over the inorganic devices currently available in the market. [Link to reference 1] Photovoltaic devices utilize photoinduced electron transfer events to convert sunlight into energy. Nonetheless, photoinduced electron transfer is not the only important process involved in this complex transformation. In this regard, the three basic steps to achieve power from sunlight are:
- 1.- Light absorption, and formation of the exciton.
- 2.- Fast and efficient electron transfer yielding the corresponding electron-hole pair, that is, the charge carriers,
- 3.- Transport of those charge carriers toward the electrodes.
The sandwich-like architecture —showing all its components - of organic solar cells is depicted in Figure 1:
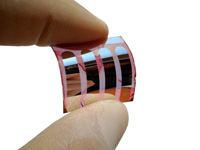
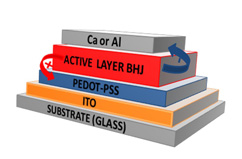
Figure 1. (left) Flexible organic solar cells (image courtesy of Prof. V. Dyakonov (Würtzburg University, Germany)); (right) Typical sandwich-like architecture of organic solar cells.
Regarding the photoactive layer in all organic solar cells, the most successful strategy utilized to obtain high PV performances is the bulk-heterojunction (BHJ).[Link to reference 2] In those organic solar cells, the active layer is formed by an interpenetrating network of a p-type material —usually a semiconducting polymer and a soluble fullerene derivative—the most used one is PCBM— acting as n-type material. This strategy has already reached power conversion efficiencies higher than 9%.[Link to reference 3]
We have recently reported the synthesis and the photovoltaic properties of a new family of soluble C60-based derivatives, called DPMs, which show outstanding power efficiency conversion values of around 2.3% in blends with P3HT as electron donor (Figure 2). [Link to reference 4] Stimulated by an idea to combine good electron accepting and light harvesting properties, we have introduced recently a new class of fullerene-based materials, constituted by two covalently connected fullerene units (C60 and/or C70). [Link to reference 5] The resulting dimers exhibit increased light-harvesting properties, due to the presence of two units of identical or different fullerenes linked through a 2-pyrazoline-pyrrolidine rings (Figure 2). Photovoltaic devices prepared with blends of these materials and P3HT exhibited promising external quantum and power conversion efficiencies.
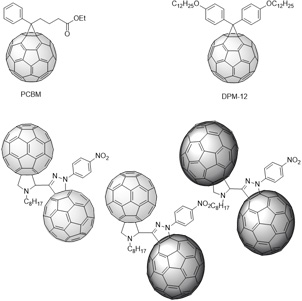
Figure 2. Chemical structure of PCBM, DPM (top) and [60]- and [70]fullerene-based dimers (down).
We have recently reported the preparation of a new cyanine-cyanine organic salt displaying exceptional light harvesting properties in the NIR spectral range. Preliminary results of molecular bulk heterojunction solar cells based on this compound and [60]PCBM as the only active layer, revealed this new dye as a promising light-harvesting material for photovoltaics. [Link to reference 6]
We have also designed and synthesized a great variety of donor and acceptor light harvesting materials, displaying outstanding extinction coefficients which could be used to prepare efficient PV devices. [Link to reference 7]
Dye Sensitized Solar Cells
The preparation of low-cost photovoltaic devices based on organic or hybrid materials, and in particular the development of metal-free organic sensitizers for dye-sensitized solar cells (DSSCs) is a challenging research field for the scientific community. [Link to reference 8]Recently, we have reported the use of the π-extended tetrathiafulvalene (exTTF) fragment for the development of new sensitizers for DSSCs. [Link to reference 9] Indeed, the butterfly shape of exTTF (Figure 3a), that may prevent aggregation, together with its exceptional electron-donor character, are making it an excellent candidate for the molecular engineering of sensitizers. Due to their redox properties, these compounds showed surprising kinetic behavior: indeed, even though the measured driving force for regeneration of the sensitizer by the redox mediator (I2/I3-) was small (150 mV), an efficient regeneration could be observed. Photovoltaic devices with a power conversion efficiency of 3.8% (AM 1.5G) were fabricated (Figure 3c). This proof-of-concept showed that sensitizers with a small driving force can efficiently operate in DSSCs.
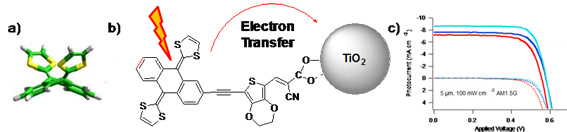
Figure 3: a) exTTF; b) Electron transfer between an exTTF-based dye and TiO2; c) corresponding photocurrent-voltage curve.
Continuing with our research on DSSC, he have recently reported the synthesis, characterization and application of new dye molecules based on the 10-(1,3-dithiol-2-ylidene)anthracene core [Link to reference 10]. Optical and electrochemical characterizations of the new materials satisfied the criteria for successful incorporation into PV devices, moreover devices fabricated with these dyes exhibited PCE around 4 %.
Hybrid Organo-Inorganic Lead Halid Perovskites Solar Cells (PSCs)
Since its first use as light absorber in a sensitized solar cell by Miyasaka and co-workers, organic-inorganic methylammonium (MA) lead halide MAPbX3 (X = I, Br) perovskites have experienced a scientific research blast for photovoltaic applications. Organometal trihalide perovskites exhibit exceptional intrinsic properties such as light absorption from visible to near-infrared range, high extinction coefficient, long electron-hole diffusion lengths, a direct band gap as well as high charge carrier mobilities, among others. Furthermore, the perovskite material is relatively versatile and its electronic properties can be widely tuned by cationic or anionic substitution. As an example, the formamidinium (FA) based perovskite FAPbI3 shows excellent light harvesting properties due to its lower band gap energy (Eg), whereas by using the methylammonium mixed halide perovskite MAPbIxBr3-x higher Eg and open circuit voltage (Voc) can be obtained. Improved energy conversion efficiencies are also observed when combining the two perovskite materials in the compositional modification (FAPbI3)1-x(MAPbBr3)x that was recently presented by Seok et al., reporting power conversion efficiencies (PCEs) above 20 %. The ambipolar behavior of the perovskite allows its combination either n-i-p or p-i-n configurations with electron transporting (ETMs) and/or with hole-transporting (HTMs) materials.
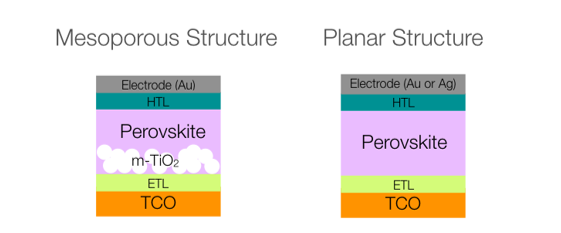
Figure 1. Most typical architectures for perovskites based solar cells.
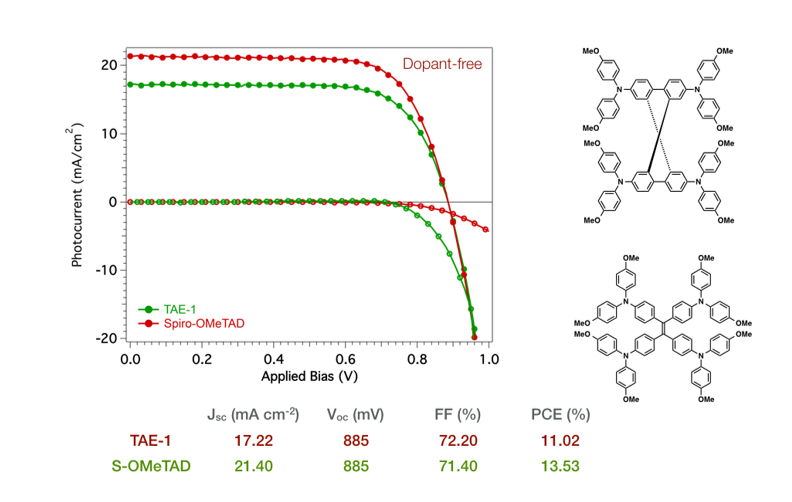
We have recently prepared small-molecule hole transporting materials based on tetraphenylethene derivatives, decorated with electron-donating diphenylamine (figure 2). Diphenylamines derivatives are one of the most used moieties in HTMs because of their three-dimensional propeller structure, which is beneficial for improving solution processability, strong electron-donor properties and excellent hole-injection and transport properties via radical cation species. The solar cells show light-to energy conversion efficiencies as high as 11% under standard measurement conditions without the need of additional dopants.11
We have also focused on the development of a new family of ease to prepare HTMs based on a benzo[1,2-b:3,4-b´:5,6-b´´]trithiophene (BTT) as a central core endowed with p-methoxydiphenylamine (BTT-1), p-methoxydiphenylamine-substituted carbazole (BTT-2) and p-methoxytriphenylamine (BTT-3) (Figure 3). BTT core exhibits a C3h symmetry with three thiophene rings fused to a benzene central ring. The planarized star-shaped structure of the BTT is foreseen to promote an effective π-π intermolecular interaction, which could eventually lead to efficient hole-transporting properties. Power conversion efficiencies in the range of 16 % to 18.2 % were achieved under AM 1.5 sun with the three derivatives tested on solution processed lead trihalide perovskite based solar cells.12
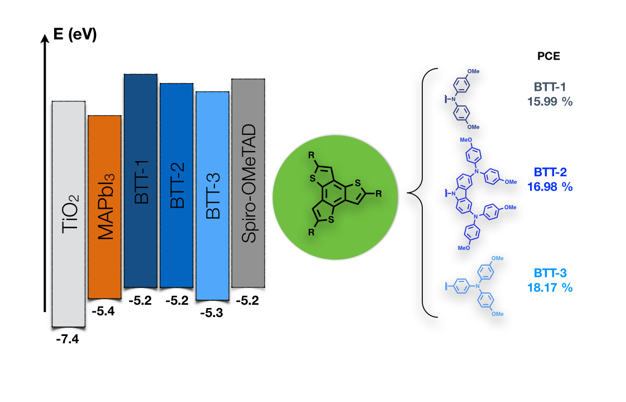
Figure 3. Chemical structures of BTT-1, BTT-2 and BTT-3 and their photovoltaic PCE values.
References
- 1. For recent reviews on PV cells, see: (a) G. Dennler, M. C. Scharber and C. J. Brabec, Adv. Mater. 2009, 21, 1; (b) B. Kippelen and J.-J. Brédas, Energy Environ. Sci. 2009, 2, 251; (c) B. C. Thompson and J. M. J. Fréchet, Angew. Chem. Int. Ed. 2008, 47, 58; (d) S. Gunes, H. Neugebauer, N. S. Sariciftci, Chem. Rev. 2007, 107, 1324; (e) J. L. Delgado, P.-A. Bouit, M. A. Herranz, S. Filipone, N. Martín, Chem. Commun, 2010, 46, 4853.
- 2. a) G. Yu, J. Gao, J. C. Hummelen, F. Wudl, A. J. Heeger, Science 1995, 270, 1789; b) A. Shah, P. Torres, R. Tscharner, N. Wyrsch, H. Keppner, Science 1999, 285, 692; c) J. Nelson, Science 2001, 293, 1059; d) C. J. Brabec, A. Cravino, D. Meissner, N. S. Sariciftci, T. Fromherz, M. T. Rispens, L. Sánchez, J. C. Hummelen, Adv. Funct. Mater. 2001, 11, 374; e) A. Gadisa, M. Svensson, M. R. Andersson, O. Inganäs, Appl. Phys. Lett. 2004, 84, 1609.
- 3. J. You, L. Dou, K. Yoshimura, T. Kato, K. Ohya, T. Moriarty, K. Emery, C.-C. Chen, J. Gao, G. Li, Y. Yang, Nat. Commun., 2013, 4, 1446
- 4. a) I. Riedel, E. von Hauff, J. Parisi, N. Martín, F. Giacalone, V. Dyakonov, Adv. Funct. Mater. 2005, 15, 1979; b) A. Sánchez-Díaz, M. Izquierdo, S. Filippone, N. Martin, E. Palomares, Adv. Funct. Mater. 2010, 16, 2695; c) G. Garcia-Belmonte, P. P. Boix, J. Bisquert, M. Lenes, H. J. Bolink, A. La Rosa, S. Filippone, and N. Martín, J. Phys. Chem. Lett., 2010, 1, 2566; d) H. J. Bolink, E. Coronado, A. Forment-Aliaga, M. Lenes, A. La Rosa, S. Filippone and N. Martín, J. Mater. Chem., 2011, 21, 1382.
- 5. a) J. L. Delgado, E. Espildora, M. Liedtke, A. Sperlich, D. Rauh, A. Baumann, C. Deibel, V. Dyakonov, N. Martin, Chem.-Eur. J.2009, 15, 13474; b) Oleg G. Poluektov, J. Niklas, K. L. Mardis, S. Beaupré, M. Leclerc, C. Villegas, S. Erten-Ela, J. L. Delgado, N. Martín, A. Sperlich, V. Dyakonov, Adv. Energy Mat., 2014, DOI: 10.1002/aenm.201301517.
- 6. P.-A. Bouit, D. Rauh, S. Neugebauer, J. L. Delgado, E. Di Piazza, S. Rigaut, O. Maury, C. Andraud, V. Dyakonov and N. Martín, Org. Lett. 2009, 11, 4806.
- 7.a) P.-A. Bouit, C. Villegas, J. L. Delgado, P. Viruela, R. Pou-Amérigo, E. Ortí, N. Martín, Org. Lett. 2011, 13, 604; b) P.-A. Bouit, L. Infantes, J. Calbo, R. Viruela, E. Ortí, J. L. Delgado, and N. Martín, Org. Lett. 2013, 15, 4166; c) C. Villegas, J. L. Delgado, P.-A. Bouit, B. Grimm, W. Seitz, N. Martin, D. M. Guldi, Chem. Sci. 2011, 2, 1677; d) P.-A. Bouit, F. Spänig, G. Kuzmanich, E. Krokos, C. Oelsner, M. A. Garcia-Garibay, J. L. Delgado, N. Martin, D. M. Guldi,Chem.-Eur. J. 2010, 16, 9638 ; e) C. Villegas, E. Krokos, P.-A. Bouit, J. L. Delgado, D. M. Guldi, N. Martin, Energy Environ. Sci., 2011, 4, 679.
- 8. a) M. Graetzel, Acc. Chem. Res. 2009, 42, 1788; b) M. Graetzel, Inorg. Chem. 2005, 44, 6841.
- 9. S. Wenger, P.-A. Bouit, Q. Chen, J. Teuscher, D. Di Censo, R. H.-Baker, J.-E. Moser, Juan L. Delgado, N. Martin, S. M. Zakeeruddin and M. Graetzel, J. Am. Chem. Soc. 2010, 132, 5164.
- 10. P.-A Bouit, M. Marszalek, R. Humphry-Baker, R. Viruela, E. Ortí, S. M. Zakeeruddin, M. Grätzel, J. L. Delgado, N. Martín,Chem. Eur. J. 2012, 18, 11621.
- 11. Cabau, L.; Garcia-Benito, I.; Molina-Ontoria, A.; Montcada, N. F.; Martin, N.; Vidal-Ferran, A.; Palomares, E., Chem. Commun. 2015, 51, 13980.
- 12. Molina-Ontoria, A.; Zimmermann, I.; Garcia-Benito, I.; Gratia, P.; Roldán-Carmona, C.; Aghazada, S.; Graetzel, M.; Nazeeruddin, M. K.; Martín, N., Angew. Chem. Int. Ed. 2016, 55, 6270.
