Fullerenes as biomolecules
Fullerenes as Biomolecules
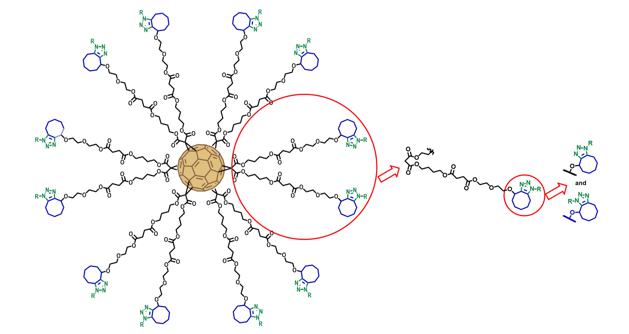
Our group has recently initiated a research line to explore the potential biological and pharmacological properties of fullerenes. The first objective is to obtain suitably functionalized fullerenes compatible with life systems. Owing to their rigid spherical shape, fullerene hexakis-adducts with a Th-symmetrical octahedral addition pattern appear to be a very attractive core molecule for the synthesis of unique globular multivalent derivatives.
Employing an approach based on click chemistry, we have efficiently prepared hexakis-adducts of fullerene decorated with sugar residues.1
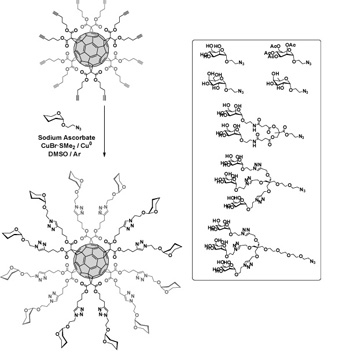
Synthesis of hexakis-adducts of fullerenes by click chemistry.
A carbohydrate–protein interaction is a key step in many biological processes. This interaction is highly selective, calcium dependent in some cases, and multivalent. The glycans are the ligands responsible to interact, in a multivalent manner, with cell surface receptors (lectins). We have studied the multivalent effect shown by these hexakis-adducts of fullerene by isothermal titration calorimetry (ITC), employing concanavalin A (Con A) as a lectin that recognizes mannoses.2 These calorimetric studies allow us to demonstrate that fullerene is a unique and rather efficient platform for the multivalent presentation of ligands, leading to a remarkable increase of affinity with a simple multivalent model.
With the aim to know the efficiency of these mannosylated systems in cellular experiments, we addressed an infection assay using Ebola virus glycoprotein (EboGP) pseudotyped viral particles as infectious agents.3 In this experimental model, the Ebola pseudovirus is absolutely dependent on interaction with DC-SIGN for viral entry and infection. The antiviral activity obtained for the hexaadduct with 36 mannoses was in the micromolar range (IC50 ~ 0.3 mM), which demonstrates that these glycofullerenes are adequate to inhibit efficiently an infection process and, therefore, to interfere in biological events where lectins such as DC-SIGN are involved.
Inhibition of infection by pseudotyped Ebola virus of Jurkat DC-SIGN+ cells using mannosylated glycofullerenes.
In the order to improve the molecular design of these glycofullerenes, we have synthesized giant globular multivalent systems in which a central C60 core is covalently connected with other 12 hexakis-adducts of fullerene to form the first tridecafullerenes reported so far.4 These globular multivalent systems were studied in the previously mentioned cellular assay and showed to inhibit the EBOV infection in a subnanomolar concentration range (IC50 ~ 0.66 nM) thus demonstrating that fullerenes with a globular presentation of ligands are appealing platforms for the study of multivalent interactions.
Tridecafullerenes are giant multivalent fullerene derivatives which efficiently inhibit infection by an artificial Ebola virus.
More recently, we have designed a new cyclooctyne appended hexakis-adduct of fullerene as a building block to carry out the copper-free SPAAC (strain promoted alkyne-azide cycloaddition) click reaction.5 This methodology avoids the use of copper as a catalyst in the click cycloaddition, being of special interest for the preparation of fullerene derivatives for biological applications.
Cyclooctyne substituted hexakis-adduct of fullerene as building block for copper-free strain
promoted alkyne-azide cycloaddition (SPAAC) reaction.
References
1. J.-F. Nierengarten, J. Iehl, V. Oerthel, M. Holler, B. M. Illescas, A. Muñoz, N. Martín, J. Rojo, M. Sánchez-Navarro, S. Cecioni, S. Vidal, K. Buffet, M. Durka, S. P. Vincent, Chem. Commun., 2010, 46, 3860.
2. M. Sánchez-Navarro, A. Muñoz, B. M. Illescas, J. Rojo, N. Martín, Chem. Eur. J., 2011, 17, 766.
3. J. Luczkowiak, A. Muñoz, M. Sánchez-Navarro, R. Ribeiro-Viana, A. Ginieis, B. M. Illescas, N. Martín, R. Delgado, J. Rojo, Biomacromolecules 2013, 14, 431.
4. A. Muñoz, D. Sigwalt, B. M. Illescas, J. Luczkowiak, L. Rodríguez-Pérez, I. Nierengarten, M. Holler, J.-S. Remy, K. Buffet, S. P. Vincent, J. Rojo, R. Delgado, J.-F. Nierengarten, N. Martín, Nat Chem, 2016, 8, 50.
5. J. Ramos-Soriano, J. J. Reina, A. Pérez-Sánchez, B. M. Illescas, J. Rojo, N. Martín, Chem. Commun., 2016, 52, 10544.