Chiral Allotropes of Carbon: Asymmetric Catalysis on Fullerenes
Chiral Allotropes of Carbon: Asymmetric Catalysis on Fullerenes
The reactivity of fullerenes when using as starting materials in several cycloadditions has been deeply investigated over the last years in our research group.1We have recently gone one step further in the evaluation of the carbon cage as a potential interesting substrate for unexplored chiral reactions.
Thus, chirality in fullerenes has recently proved to play a critical role in the control of some physical properties. The difficult activation and the even more difficult asymmetric induction of all-carbon cages in fullerene science have strongly limited the use of chiral fullerene derivatives and, therefore, the development of chiral carbon nanostructures with enhanced properties. The absence of functional groups and the no coordinating polyolefin nature of fullerenes prevent both the use of metal mediated processes and the employ of chiral organic molecules to activate the fullerene double bonds.
However, we succeeded in reporting the first synthesis of chiral pyrrolidinofullerenes prepared “a la carta” by metal catalyzed enantioselective 1,3-dipolar cycloaddition of N-metalated azomethine ylides.2 The stereochemical outcome of the reaction is possible by modifying the metal catalyst [Ag(I) or Cu(II)] in combination with a variety of chiral ligands.3 An enantioselective process was also found in the retrocycloaddition reaction as revealed by mass spectrometry analysis on quasi-enantiomeric pyrrolidino[60]fullerenes. Theoretical DFT calculations are in very good agreement with the experimental data.4
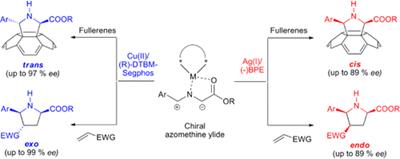
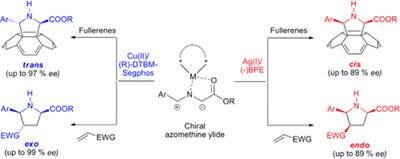
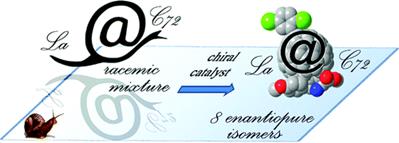
Moreover, due to the growing interest of higher fullerenes, this methodology has also been successfully extended to higher fullerenes, namely C705 as well as to metallofullerenes, namely La@C72(C6H3Cl2).6 The high degree of stereocontrol achieved demonstrates that this methodology is able to face different levels of selectivity and stereocontrol, affording either the cis or trans adducts with excellent enantiomeric excesses.
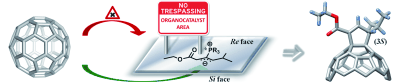
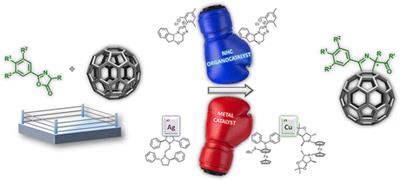
Organocatalytic methods have also been applied for the first time in fullerene science affording chiral [60]fullerene derivatives by a convergent phosphine-catalyzed [3+2] cycloaddition of allenoates onto C60.7 More recently our group have merge both metal and organocatalytic methodologies for the development of enantioselective [3+2] cycloaddition of münchnones onto fullerenes and olefins.8
Recently, we have exploited the chiral information introduced onto of endohedrals obtained by the so called “molecular surgery” methods, such as H2@C60 and H2O@C60, to carry out a mechanistic study onto the cis-trans isomerization. Experimental results and computational calculations revealed the favorable effect of the H-bonding in stabilizing the intermediate carbanion by the inner water molecule encapsulated in the fullerene cage. 9
References
1. a) Martín, N.; Altable, M.; Filippone, S.; Martín-Domenech, A. Chem. Commun. 2004, 1338. b) Martín, N.; Altable, M.; Filippone, S.; Martín-Domenech, A.; Poater, A.; Solá, M. Chem. Eur. J. 2005, 11, 2716. c) Altable, M.; Filippone, S.; Martín-Domenech, A.; Güell, M.; Solá, M.; Martín, N. Org. Lett. 2006, 8, 5959. d) Martín, N. Altable, M.; Filippone, S.; Martín-Domenech, A.; Güell, M.; Solá, M.; Angew. Chem. Int. Ed. 2006, 45, 1439. e) Martín, N.; Altable, M.; Filippone, S.; Martín-Domenech, A.Echegoyen, L.; Cardona, C. M. Angew. Chem. Int. Ed. 2006, 45, 110. f) Lukoyanova, O.; Cardona, C. M.; Altable, M.; Filippone, S.; Martín-Domenech, A.; Martín, N.; Echegoyen, L. Angew. Chem. Int. Ed. 2006, 45, 7430. g) Filippone, S.; Izquierdo, M.; Martín-Domenech, A.; Osuna, S.; Solá, M.; Martín, N. Chem. Eur. J. 2008, 14, 5198. h) Izquierdo, M.; Osuna, S.; Filippone, S.; Martín-Domenech, A.; Solá, M.; Martín, N. J. Org. Chem. 2009, 74, 1480.i) Izquierdo, M.; Osuna, S.; Filippone, S.; Martín-Domenech, A.; Solá, M.; Martín, N. J. Org. Chem. 2009, 74, 6253. j) Izquierdo, M.; Osuna, S.; Filippone, S.; Martín-Domenech, A.; Solá, M.; Martín, Eur. J. Org. Chem. 2009, 35, 6231.
2. Filippone, S.; Maroto, E. E.; Martín-Domenech, A.; Suárez, M.; Martín, N. Nat. Chem. 2009, 1, 578.
3. Maroto, E. E.; Filippone, S.; Martín-Domenech, A.; Suárez, M.; Martín, N. J. Am. Chem. Soc. 2012, 134, 12936.
4. Maroto, E. E.; Filippone, S.; Suárez, M.; Martínez-Álvarez, R.; de Cózar, A.; Cossío, F. P.; Martín, N. J. Am. Chem. Soc. 2014, 136, 705.
5. Maroto, E. E.; de Cózar, A.; Filippone, S.; Martín-Domenech, A.; Suárez, M.; Cossío, F. P.; Martín, N. Angew. Chem., Int. Ed. 2011, 50, 6060.
6. Sawai, K.; Takano, Y.; Izquierdo, M.; Filippone, S.; Martín, N.; Slanina, Z.; Mizorogi, N.; Waelchli, M.; Tsuchiya, T.; Akasaka, T.; Nagase, S. J. Am. Chem. Soc. 2011, 133, 17746.
7. Marco-Martínez, J.; Marcos, V.; Reboredo, S.; Filippone, S.; Martín, N. Angew. Chem. Int. Ed. 2013, 52, 5115.
8. Marco-Martínez, J.; Reboredo, S.; Izquierdo, M.; Marcos, V.; López, J. L.; Filippone, S.; Martín, N. J. Am. Chem. Soc. 2014, 136, 2897.
9. a) E. E. Maroto, J. Mateos, M. Garcia-Borràs, S. Osuna, S. Filippone, M. Á. Herranz, Y. Murata, M. Solà, N. Martín, J. Am. Chem. Soc. 2015,137, 1190-1197; b) E. E. Maroto, M. Izquierdo, M. Murata, S. Filippone, K. Komatsu, Y. Murata, N. Martín, Chem. Commun. 2014, 50, 740; c) E. E. Maroto, M. Izquierdo, S. Reboredo, J. Marco-Martínez, S. Filippone, N. Martín "Chiral Fullerenes from Asymmetric Catalysis" Acc. Chem. Res. 2014, 47, 2660−2670.