Carbon Nanotubes:Chemical Reactivity and Properties
Carbon Nanotubes: Chemical Reactivity and Properties
Since their discovery, carbon nanotubes (CNTs) are among the materials of choice for applications in nanotechnology due to the excellent characteristics that present: chemical stability, high-quality mechanical properties, high surface area and thermal conductivity.
With the long term objective of constructing versatile and functional nanosized ensembles for photoinduced electron transfer, we embarked on the preparation of donor-acceptor nanohybrids considering the combination of carbon nanotubes with TTF-based p-type building blocks following different, covalent and non-covalent methodologies.
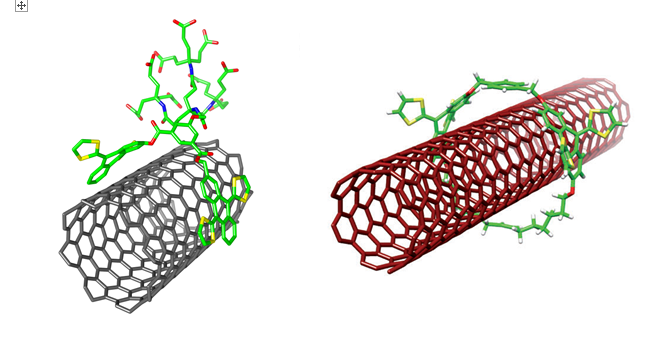
SWNTs-exTTF covalent and non-covalent nanostructures.
In a first approach, we prepared single-walled carbon nanotubes (SWNT) endowed with covalently linked tetrathiafulvalene (TTF) derivatives by using simple esterification reactions. Results from near-infrarred and transient absorption measurements showed that the charge recombination dynamics is a function of the spacer linking the TTF moiety to the nanotube and the donor ability of the different TTF derivatives investigated.1
At the fundamental level, the chemical reactivity of sp2-hybridized carbons is affected by the curvature and for CNTs exists a direct correlation between the tube diameter and reactivity. The differences between SWNTs, MWNTs and graphene have been investigated through a combination of arylation and click chemistry CuI-mediated azide–alkyne cycloaddition (CuAAC) reactions.2 Important differences in the grafting density were noticed by thermogravimetric analysis (TGA) measurements, with a significantly higher degree of functionalization for SWNTs (26%) when compared to MWNTs (8%).
Although the covalent approach is very versatile, an alternative strategy to control the organization between donor and acceptor units, while preserving the p system of the graphene sheets and, therefore, the electronic structure of CNTs, consists in the supramolecular funtionalization of SWNTs by means of hydrophobic, π-stacking, or van der Waals interactions with the sidewalls of SWNTs. For this purpose, we have prepared pyrene-exTTF3 or pyrene-TTF4 derivatives and investigated their electron donor-acceptor interactions with different types of CNTs in organic solvents. We accomplished the solubilization of HiPco and CoMoCAT SWNTs in water, considering dendronized molecules endowed with exTTF moieties (see Figure below).5
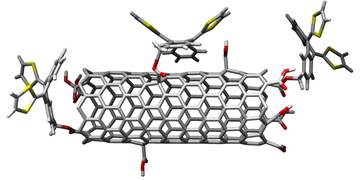
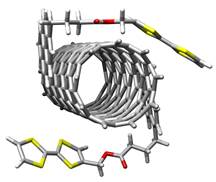
Besides the covalent and non-covalent modification of SWNTs to attain specific electronic properties, SWNTs can form mechanically interlocked species.6 In the key rotaxane-forming step, we employed macrocycle precursors equipped with two π-extended tetrathiafulvalene SWNT recognition units and terminated with bisalkenes that were closed around the nanotubes through ring-closing metathesis (RCM).
exTTF-based nanotweezer hybrids and mechanically interlocked SWNTs.
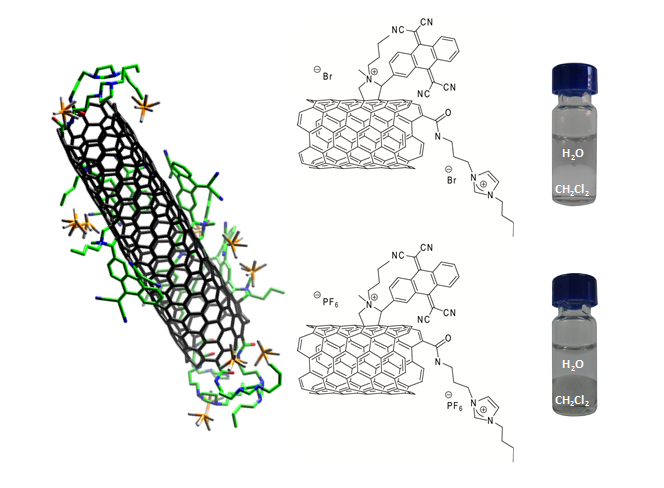
Turning to n-type building blocks, tweezer-shaped 11,11,12,12-tetracyano-9,10-anthraquinodimethane (TCAQ) molecules formed exceptionally stable n-/p-type dispersions with SWNTs in aqueous media.7 A state of the art photophysical investigation, carried out in collaboration with the group of Prof. Dirk M. Guldi (University of Erlangen, Germany) supports the notion that a dark charge-transfer state in SWNT/TCAQ nanotweezers transforms upon photoexcitation into a fully charge-separated state.When SWNTs are doubly functionalizated with an imidazolium cation-based ionic liquid, in addition to the TCAQ units, the solubility of the nanomaterial can be adjusted to different media through counteranion exchange. The obtained SWNTs present a switchable polarity between organic solvents and water.8
SWNTs incorporating n-type TCAQ units with tunable solubility.
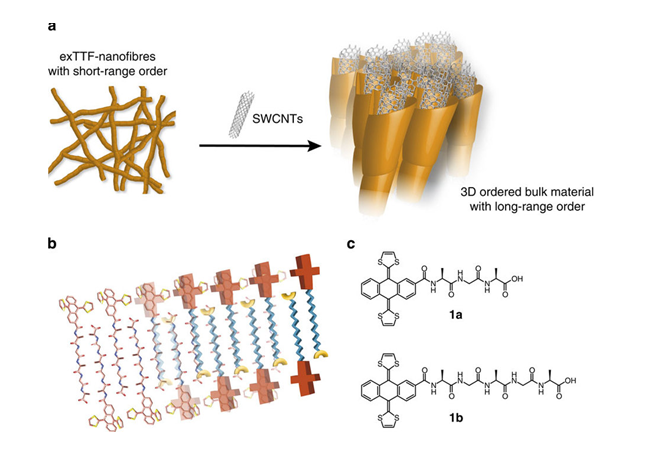
To gain control over the CNT-exTTF nanomaterials, we have designed exTTFs covalently linked to peptides of different lengths, which enable the spontaneous self-assembly of SWNTs into well-ordered structures of different scales.9 In the p-type nanofibers formed by SWNTs and tripeptide and pentapeptide-exTTFs, the self-assembly can be externally controlled by charge screening with Ca2+, resulting in new and stable SWNT-based supramolecular gels featuring remarkably long-range internal order.10
(a) Cartoon showing SWCNTs as additives for controlling the crystalline ordering in bulk materials. (b) Schematic illustration of a β-sheet secondary supramolecular structure built up by peptide-based exTTFs. (c) Peptide-based exTTFs used in this study.
References
1.- M.A. Herranz, N. Martín, S. Campidelli, M. Prato, G. Brehm, D.M. Guldi, Angew. Chem. Int. Ed. 2006, 45, 4478-4482.
2.- J. Mateos-Gil, L. Rodríguez-Pérez, M. Moreno Oliva, G. Katsukis, C. Romero-Nieto, M.A. Herranz, D.M. Guldi, Nanoscale, 2015, 7, 1193-1200.
3- . M.A. Herranz, C. Ehli, S. Campidelli, M. Gutiérrez, G.L. Hug, K. Ohkubo, S. Fukuzumi, M. Prato, N. Martín, D.M. Guldi, J. Am. Chem. Soc. 2008,130, 66-73.
4.- a) C. Ehli, D.M. Guldi, M.A. Herranz, N. Martín, S. Campidelli, M. Prato, J. Mater. Chem. 2008, 18, 1498-1503; b) A. Wurl, S. Goossen, D. Canevet, M. Sallé, E.M. Pérez, N. Martín, C. Klinke, J. Phys. Chem. C 2012, 116, 20062-2006.
5.- C. Romero-Nieto, R. García, M.A. Herranz, C. Ehli, M. Ruppert, A. Hirsch, D.M. Guldi, N. Martín, J. Am. Chem. Soc. 2012, 134, 9183-9192.
6.- A. de Juan,Y. Pouillon, L. Ruiz-González, A. Torres-Pardo, S. Casado, N. Martín, A. Rubio, E.M. Pérez, Angew. Chem. Int. Ed. 2014, 53, 5498-5504.
7.- C. Romero-Nieto, R. García, M.A. Herranz, L. Rodríguez-Pérez, M. Sánchez-Navarro, J. Rojo, N. Martín, D.M. Guldi, Angew. Chem. Int. Ed. 2013,52, 10216-10220.
8.- . L. Rodríguez-Pérez, R. García, M.A. Herranz, N. Martín, Chem. Eur. J. 2014, 20, 7278-7286.
9.- F.G. Brunetti, C. Romero-Nieto, J. López-Andarias, C. Atienza, J.L. López, D.M. Guldi, N. Martín, Angew. Chem. Int. Ed. 2013, 52, 2180-2184.
10.- J. López-Andarias, J.L. López, C. Atienza, F.G. Brunetti, C. Romero-Nieto, D.M. Guldi, N. Martín, Nature Communications, 2014, 5, 3763.