Lines of Research
Background
Previously, the PI studied the signal transduction pathways of the WASP protein, so named because it is mutated in the immunodeficiency called Wiskott-Aldrich syndrome. Thus it was found that WASP-interacting protein (WIP) is an inhibitor of N-WASP activity [1] and in collaboration with Dr. Fred Alt, detailed studies of WAVE2, another member of the WASP family of proteins were carried out [2].
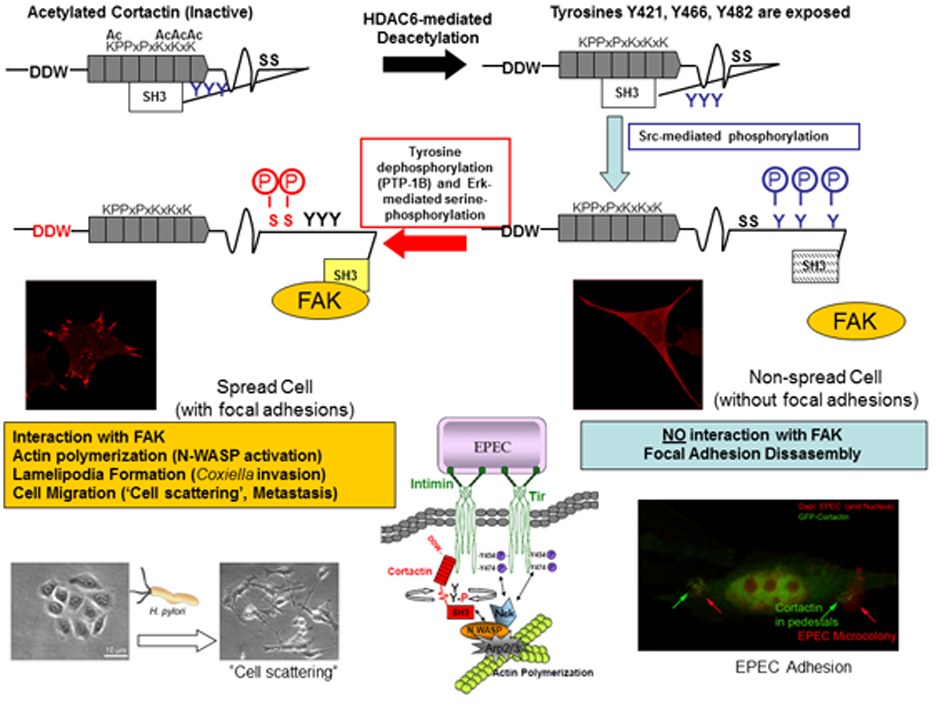
A key seminal contribution to research on the cytoskeleton is the demonstration that cortactin is a potent activator of N-WASP, thereby uncovering a connection between the two major families of activators of the Arp2/3 complex. Furthermore, an on-off switching mechanism regulated by Erk and Src phosphorylation of cortactin was proposed [3].The model has since been featured in various reviews and commentaries [4-7]. This model, called the “S-Y switch" has been studied in diverse fields [8, 9] and was the start point for the research lines in our group.
Lines of research
In 2005, our group initiated completely independent lines of research to study cortactin regulation using the model of infection enteropathogenic Escherichia coli (EPEC), which adheres to cells by forming actin pedestals [10, 11]. During this period, we established collaborations to study the role of cortactin in the infection of cells by Helicobacter pylori [12] and by Coxiella Burnetti [13].
Another line of research developed has been the study of the regulation of cortactin by post-translational modifications and its role in cell spreading [14]. Thus we have characterized a novel cortactin-FAK complex that functions as a “molecular clutch” in integrin activation [12, 14]. In addition, we found a competettion between the acetylation and tyrosine phosphorylation of cortactin and that phosphorylation inhibits cell spreading. Furthermore, we demonstrate that cell spreading promotes the association of cortactin and FAK and that tyrosine phosphorylation of cortactin disrupts this interaction, which may explain how it inhibits cell spreading [14].
Cortactin favours the polymerization of actin in diverse processes by interacting with different proteins in its role as a scaffolding protein. This implies that insights into cortactin regulation and activity will have repercussions in many research fields such as cell migration and bacterial invasion of host cells.
On the other side, the locus encoding cortactin protein (CTTN) is located in the 11q13 region, which is amplified in human carcinomas [15]. Numerous studies suggest that overexpression of cortactin leads to increased cell migration and metastasis. In addition, phosphorylation of cortactin on tyrosine is currently under consideration as a prognostic marker [19].
Our future research plan is to continue investigating actin-based motility processes in human diseases. This includes pathways responsible for cell migration and adhesion. Currently, we have a special interest in proteins such as cortactin, the Wiskott-Aldrich protein (WASP) and adaptor proteins, including Crk and Nck.
Our recent research project is centered in the study of the regulation and signaling pathways mediated by the immune cortactin paralogue HS1 protein.
References
- Martinez-Quiles, N., et al., WIP regulates N-WASP-mediated actin polymerization and filopodium formation. Nat Cell Biol, 2001. 3(5): p. 484-91.
- Yan, C., et al., WAVE2 deficiency reveals distinct roles in embryogenesis and Rac-mediated actin-based motility. EMBO J, 2003. 22(14): p. 3602-12.
- Martinez-Quiles, N., et al., Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol Cell Biol, 2004. 24(12): p. 5269-80.
- Kelley, L.C., et al., Revisiting the ERK/Src cortactin switch. Commun Integr Biol, 2011. 4(2): p. 205-7.
- Evans, J.V., et al., Further insights into cortactin conformational regulation. Bioarchitecture, 2011. 1(1): p. 21-23.
- Kelley, L.C., et al., Cortactin phosphorylated by ERK1/2 localizes to sites of dynamic actin regulation and is required for carcinoma lamellipodia persistence. PLoS One, 2010. 5(11): p. e13847.
- Lua, B.L. and B.C. Low, Cortactin phosphorylation as a switch for actin cytoskeletal network and cell dynamics control. FEBS Lett, 2005. 579(3): p. 577-85.
- Ayala, I., et al., Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci, 2008. 121(Pt 3): p. 369-78.
- Kruchten, A.E., et al., Distinct phospho-forms of cortactin differentially regulate actin polymerization and focal adhesions. Am J Physiol Cell Physiol, 2008. 295(5): p. C1113-22.
- Brandt, S., et al., Dual infection system identifies a crucial role for PKA-mediated serine phosphorylation of the EPEC-Tir-injected effector protein in regulating Rac1 function. Cell Microbiol, 2009. 11(8): p. 1254-71.
- Nieto-Pelegrin, E. and N. Martinez-Quiles, Distinct phosphorylation requirements regulate cortactin activation by TirEPEC and its binding to N-WASP. Cell Commun Signal, 2009. 7: p. 11.
- Tegtmeyer, N., et al., Serine phosphorylation of cortactin controls focal adhesion kinase activity and cell scattering induced by Helicobacter pylori. Cell Host Microbe, 2011. 9(6): p. 520-31.
- Rosales, E.M., et al., Cortactin is involved in the entry of Coxiella burnetii into non-phagocytic cells. PLoS One, 2012. 7(6): p. e39348.
- Meiler, E., E. Nieto-Pelegrin, and N. Martinez-Quiles, Cortactin tyrosine phosphorylation promotes its deacetylation and inhibits cell spreading. PLoS One, 2012. 7(3): p. e33662.
- Schuuring, E., et al., Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene, 1992. 7(2): p. 355-61.
- Matsuo, T., et al., Pathologic significance and prognostic value of phosphorylated cortactin expression in patients with sarcomatoid renal cell carcinoma. Urology, 2011. 78(2): p. 476 e9-15.