Caprolactam production Process
Introduction
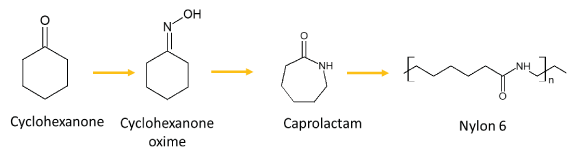
Nylon 6 is produced by ring-opening polymerization of ε-caprolactam. More than 98% of the ε-caprolactam is produced using cyclohexanone as an intermediate. Cyclohexanone is obtained either by hydrogenation of phenol, or by catalytic and non-catalytic oxidation of cyclohexane with air or by hydration of cyclohexene to cyclohexanol followed by dehydrogenation. The most common cyclohexanone production process is promoted by oxidation of cyclohexane in the presence of catalytic metal salts to obtain a reaction mixture known as KA-oil, which contains cyclohexanone, cyclohexanol and other impurities. Cyclohexanone, after being purified from KA-oil, reacts with hydroxylamine (usually added as hydroxylamine sulphate) to produce cyclohexanone oxime . In this reaction, the sulphuric acid formed is neutralized using ammonia and ammonium sulphate is obtained as by-product. To avoid the formation of ammonium sulphate, the ammoximation of cyclohexanone has been recently proposed. In the ammoximation of cyclohexanone, aqueous H2O2 and ammonia react with cyclohexanone by means of solid titanosilicate as a catalyst (known as TS-1).After oximation, the Beckmann rearrangement (BR) of cyclohexanone oxime in oleum media produces -caprolactam. However, besides -caprolactam, other by-products are formed in the Beckmann rearrangement process.
Optimization-Based Design of a Reactive Distillation Column for the Purification Process of Cyclohexanone Using Rigorous Simulation Model and Validated Using an Experimental Packed Column
Transformation of Cyclic Ketones as Impurities in Cyclohexanone in the Caprolactam Production Process
Related works:
- Lorenzo, D., Romero, A., Del-Arco, L., & Santos, A. (2019). Linear amides in Caprolactam from Linear ketone impurities in Cyclohexanone obtained from cyclohexane: kinetics and identification. Industrial & Engineering Chemistry Research. DOI: https://doi.org/10.1021/acs.iecr.9b01997
- Lorenzo, D., Romero, A., del Arco Puente, L., & Santos, A. (2019). Transformation of cyclic ketones as impurities in cyclohexanone in the caprolactam production process. Industrial & Engineering Chemistry Research. DOI: https://doi.org/10.1021/acs.iecr.9b04982
- Lorenzo, D., Santos, A., Perez-Galvan, C., Triana, C., Romero, A., & Bogle, I. D. L. (2018). Optimization-Based Design of a Reactive Distillation Column for the Purification Process of Cyclohexanone Using Rigorous Simulation Model and Validated Using an Experimental Packed Column. Industrial & Engineering Chemistry Research, 57(48), 16407-16422. DOI: https://doi.org/10.1021/acs.iecr.8b03560
- Lorenzo, D., A. Santos, E. Simon and A. Romero (2013). Kinetic of Alkali Catalyzed Self-Condensation of Cyclohexanone. Industrial & Engineering Chemistry Research. 52(6): 2257-2265.
- Lorenzo, D., A. Santos, E. Simon and A. Romero (2013). Kinetics of Alkali-Catalyzed Condensation of Impurities in the Cyclohexanone Purification Process. Industrial & Engineering Chemistry Research. 52(45): 15780-15788.
- Simon, E., J. Maria Rosas, A. Santos and A. Romero (2013). Coke formation in copper catalyst during cyclohexanol dehydrogenation: Kinetic deactivation model and catalyst characterization. Chemical Engineering Journal. 214: 119-128.
- Simon, E., J. Maria Rosas, A. Santos and A. Romero (2012). Study of the deactivation of copper-based catalysts for dehydrogenation of cyclohexanol to cyclohexanone. Catalysis Today. 187(1): 150-158.
- Simon, E., F. Pardo, D. Lorenzo, A. Santos and A. Romero (2012). Kinetic model of 2-cyclohexenone formation from cyclohexanol and 2-cyclohexenol dehydrogenation. Chemical Engineering Journal. 192: 129-137.
- Romero, A., A. Santos, G. Ruiz and E. Simon (2011). Phenol Production Kinetic Model in the Cyclohexanol Dehydrogenation Process. Industrial & Engineering Chemistry Research. 50(14): 8498-8504.
- Romero, A., A. Santos, D. Escrig and E. Simon (2011). Comparative dehydrogenation of cyclohexanol to cyclohexanone with commercial copper catalysts: Catalytic activity and impurities formed. Applied Catalysis a-General. 392(1-2): 19-27.
Collaboration with:
